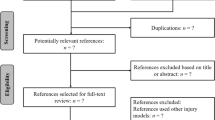
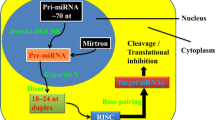
Abstract
miRNA therapy is popularly investigated in treating acute spinal cord injury (SCI) and offers a significant prospect for the treatment of acute SCI. We aimed to provide pre-clinical validations of miRNA in the treatment of SCI. A systematic search of EMBASE, PubMed, Web of Science, the Cochrane Library, and Scopus databases was performed. Rats, which were the most used animals (70%, n = 46 articles), receiving miRNA therapy got prominent recovery in SCI models [BBB score, SMD 3.90, 95% CI 3.08–4.73, p < 0.01]. Locomotor function of fore and hind limbs in SCI mice receiving miRNA therapy (30%, n = 19 articles) [grip strength, SMD 3.22, 95% CI 2.14–4.26; p < 0.01; BBB score, SMD 3.47, 95% CI 2.38–4.56, p < 0.01; BMS, SMD 2.27, 95% CI 1.34–3.20, p < 0.01] also recovered better than mice in control group. Then, we conducted the subgroup analysis and did find that high-quality articles trended to report non-therapeutic effect of miRNA. Furtherly, we analyzed 46 miRNAs, including 9 miRNA families (miR-21-5p/34a-3p/124-3p/126-3p/223-3p/543-3p/30-3p/136-3p/15-5p), among which miR-30-3p/136-3p/15-5p family were not effective in recovering locomotor function of rats. Conclusively, miRNAs are curative drugs for SCI, however, appropriate miRNA carrier and which miRNA is the most efficacious for SCI should be furtherly investigated.





Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Bushati N, Cohen SM (2007) microRNA functions. Annu Rev Cell Dev Biol 23:175–205. https://doi.org/10.1146/annurev.cellbio.23.090506.123406
Dai J, Xu LJ, Han GD, Sun HL, Zhu GT, Jiang HT, Yu GY, Tang XM (2018) MicroRNA-125b promotes the regeneration and repair of spinal cord injury through regulation of JAK/STAT pathway. Eur Rev Med Pharmacol Sci 22(3):582–589. https://doi.org/10.26355/eurrev_201802_14271
Eldahan KC, Rabchevsky AG (2018) Autonomic dysreflexia after spinal cord injury: systemic pathophysiology and methods of management. Auton Neurosci 209:59–70. https://doi.org/10.1016/j.autneu.2017.05.002
He QQ, Xiong LL, Liu F, He X, Feng GY, Shang FF, Xia QJ, Wang YC, Qiu DL, Luo CZ, Liu J, Wang TH (2016) MicroRNA-127 targeting of mitoNEET inhibits neurite outgrowth, induces cell apoptosis and contributes to physiological dysfunction after spinal cord transection. Sci Rep 6:35205. https://doi.org/10.1038/srep35205
Hede K (2013) Emergency medicine: the need for speed. Nature 503(7475):S14-15. https://doi.org/10.1038/503S14a
Hu Y, Liu Q, Zhang M, Yan Y, Yu H, Ge L (2019) MicroRNA-362-3p attenuates motor deficit following spinal cord injury via targeting paired box gene 2. J Integr Neurosci 18(1):57–64. https://doi.org/10.31083/j.jin.2019.01.12
Huang X, Gu YK, Cheng XY, Su ZD (2017) Astrocytes as therapeutic targets after spinal cord injury. Sheng Li Xue Bao 69(6):794–804
Huang JH, Xu Y, Yin XM, Lin FY (2020) Exosomes derived from miR-126-modified MSCs promote angiogenesis and neurogenesis and attenuate apoptosis after spinal cord injury in rats. Neuroscience 424:133–145. https://doi.org/10.1016/j.neuroscience.2019.10.043
Jee MK, Jung JS, Choi JI, Jang JA, Kang KS, Im YB, Kang SK (2012) MicroRNA 486 is a potentially novel target for the treatment of spinal cord injury. Brain 135(Pt 4):1237–1252. https://doi.org/10.1093/brain/aws047
Jiang D, Gong F, Ge X, Lv C, Huang C, Feng S, Zhou Z, Rong Y, Wang J, Ji C, Chen J, Zhao W, Fan J, Liu W, Cai W (2020) Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J Nanobiotechnology 18(1):105. https://doi.org/10.1186/s12951-020-00665-8
Koda M, Nishio Y, Kamada T, Someya Y, Okawa A, Mori C, Yoshinaga K, Okada S, Moriya H, Yamazaki M (2007) Granulocyte colony-stimulating factor (G-CSF) mobilizes bone marrow-derived cells into injured spinal cord and promotes functional recovery after compression-induced spinal cord injury in mice. Brain Res 1149:223–231. https://doi.org/10.1016/j.brainres.2007.02.058
Kumar B, Pandey M, Fayaz F, Izneid TA, Pottoo FH, Manchanda S, Sharma A, Sahoo PK (2020) Applications of exosomes in targeted drug delivery for the treatment of Parkinson’s disease: a review of recent advances and clinical challenges. Curr Top Med Chem 20(30):2777–2788. https://doi.org/10.2174/1568026620666201019112557
Li R, Yin F, Guo Y, Ruan Q, Zhu Q (2018) Angelica polysaccharide protects PC-12 cells from lipopolysaccharide-induced injury via down-regulating microRNA-223. Biomed Pharmacother 108:1320–1327. https://doi.org/10.1016/j.biopha.2018.09.147
Li R, Bao L, Hu W, Liang H, Dang X (2019a) Expression of miR-210 mediated by adeno-associated virus performed neuroprotective effects on a rat model of acute spinal cord injury. Tissue Cell 57:22–33. https://doi.org/10.1016/j.tice.2019.02.004
Li XZ, Lv CL, Shi JG, Zhang CX (2019b) MiR-543-3p promotes locomotor function recovery after spinal cord injury by inhibiting the expression of tumor necrosis factor superfamily member 15 in rats. Eur Rev Med Pharmacol Sci 23(7):2701–2709. https://doi.org/10.26355/eurrev_201904_17540
Li C, Li X, Zhao B, Wang C (2020) Exosomes derived from miR-544-modified mesenchymal stem cells promote recovery after spinal cord injury. Arch Physiol Biochem 126(4):369–375. https://doi.org/10.1080/13813455.2019.1691601
Liu D, Huang Y, Jia C, Li Y, Liang F, Fu Q (2015) Administration of antagomir-223 inhibits apoptosis, promotes angiogenesis and functional recovery in rats with spinal cord injury. Cell Mol Neurobiol 35(4):483–491. https://doi.org/10.1007/s10571-014-0142-x
Lu TX, Rothenberg ME (2018) MicroRNA. J Allergy Clin Immunol 141(4):1202–1207. https://doi.org/10.1016/j.jaci.2017.08.034
Mohr AM, Mott JL (2015) Overview of microRNA biology. Semin Liver Dis 35(1):3–11. https://doi.org/10.1055/s-0034-1397344
Nieto-Diaz M, Esteban FJ, Reigada D, Muñoz-Galdeano T, Yunta M, Caballero-López M, Navarro-Ruiz R, Del Águila A, Maza RM (2014) MicroRNA dysregulation in spinal cord injury: causes, consequences and therapeutics. Front Cell Neurosci 8:53. https://doi.org/10.3389/fncel.2014.00053
Ning SL, Zhu H, Shao J, Liu YC, Lan J, Miao J (2019) MiR-21 inhibitor improves locomotor function recovery by inhibiting IL-6R/JAK-STAT pathway-mediated inflammation after spinal cord injury in model of rat. Eur Rev Med Pharmacol Sci 23(2):433–440. https://doi.org/10.26355/eurrev_201901_16852
Park ES, Ahn JM, Jeon SM, Cho HJ, Chung KM, Cho JY, Youn DH (2017) Proteomic analysis of the dorsal spinal cord in the mouse model of spared nerve injury-induced neuropathic pain. J Biomed Res 31(6):494–502. https://doi.org/10.7555/jbr.31.20160122
Qi L, Jiang-Hua M, Ge-Liang H, Qing C, Ya-Ming L (2019) MiR-34a inhibits spinal cord injury and blocks spinal cord neuron apoptosis by activating phatidylinositol 3-kinase (PI3K)/AKT pathway through targeting CD47. Curr Neurovasc Res 16(4):373–381. https://doi.org/10.2174/1567202616666190906102343
Rodrigues LF, Moura-Neto V, Spohr TCLS (2018) Biomarkers in spinal cord injury: from prognosis to treatment. Mol Neurobiol 55(8):6436–6448. https://doi.org/10.1007/s12035-017-0858-y
Shi Z, Zhou H, Lu L, Li X, Fu Z, Liu J, Kang Y, Wei Z, Pan B, Liu L, Kong X, Feng S (2017) The roles of microRNAs in spinal cord injury. Int J Neurosci 127(12):1104–1115. https://doi.org/10.1080/00207454.2017.1323208
Song JL, Zheng W, Chen W, Qian Y, Ouyang YM, Fan CY (2017) Lentivirus-mediated microRNA-124 gene-modified bone marrow mesenchymal stem cell transplantation promotes the repair of spinal cord injury in rats. Exp Mol Med 49(5):e332. https://doi.org/10.1038/emm.2017.48
Sun P, Liu DZ, Jickling GC, Sharp FR, Yin KJ (2018) MicroRNA-based therapeutics in central nervous system injuries. J Cereb Blood Flow Metab 38(7):1125–1148. https://doi.org/10.1177/0271678x18773871
Tao B, Shi K (2016) Decreased miR-195 expression protects rats from spinal cord injury primarily by targeting HIF-1α. Ann Clin Lab Sci 46(1):49–53
Theis T, Yoo M, Park CS, Chen J, Kügler S, Gibbs KM, Schachner M (2017) Lentiviral delivery of miR-133b improves functional recovery after spinal cord injury in mice. Mol Neurobiol 54(6):4659–4671. https://doi.org/10.1007/s12035-016-0007-z
Wan G, An Y, Tao J, Wang Y, Zhou Q, Yang R, Liang Q (2020) MicroRNA-129-5p alleviates spinal cord injury in mice via suppressing the apoptosis and inflammatory response through HMGB1/TLR4/NF-κB pathway. Biosci Rep 40(3):BSR20193315. https://doi.org/10.1042/bsr20193315
Wang Y, Sun JC, Wang HB, Xu XM, Yang Y, Kong QJ, Shi JG (2018) Effects of MicroRNA-494 on astrocyte proliferation and synaptic remodeling in the spinal cord of a rat model of chronic compressive spinal cord injury by regulating the Nogo/Ngr signaling pathway. Cell Physiol Biochem 48(3):919–933. https://doi.org/10.1159/000491959
Wang B, Shen PF, Qu YX, Zheng C, Xu JD, Xie ZK, Cao XJ (2019) miR-940 promotes spinal cord injury recovery by inhibiting TLR4/NF-κB pathway-mediated inflammation. Eur Rev Med Pharmacol Sci 23(8):3190–3197. https://doi.org/10.26355/eurrev_201904_17677
Wang Y, Lai X, Wu D, Liu B, Wang N, Rong L (2021) Umbilical mesenchymal stem cell-derived exosomes facilitate spinal cord functional recovery through the miR-199a-3p/145-5p-mediated NGF/TrkA signaling pathway in rats. Stem Cell Res Ther 12(1):117. https://doi.org/10.1186/s13287-021-02148-5
Wu WD, Wang LH, Wei NX, Kong DH, Shao G, Zhang SR, Du YS (2019) MicroRNA-15a inhibits inflammatory response and apoptosis after spinal cord injury via targeting STAT3. Eur Rev Med Pharmacol Sci 23(21):9189–9198. https://doi.org/10.26355/eurrev_201911_19409
Xie W, Yang SY, Zhang Q, Zhou Y, Wang Y, Liu R, Wang W, Shi J, Ning B, Jia T (2018) Knockdown of MicroRNA-21 promotes neurological recovery after acute spinal cord injury. Neurochem Res 43(8):1641–1649. https://doi.org/10.1007/s11064-018-2580-1
Xu Z, Zhang K, Wang Q, Zheng Y (2019) MicroRNA-124 improves functional recovery and suppresses Bax-dependent apoptosis in rats following spinal cord injury. Mol Med Rep 19(4):2551–2560. https://doi.org/10.3892/mmr.2019.9904
Xu Z, Zeng S, Gong Z, Yan Y (2020) Exosome-based immunotherapy: a promising approach for cancer treatment. Mol Cancer 19(1):160. https://doi.org/10.1186/s12943-020-01278-3
Yu T, Zhao C, Hou S, Zhou W, Wang B, Chen Y (2019) Exosomes secreted from miRNA-29b-modified mesenchymal stem cells repaired spinal cord injury in rats. Braz J Med Biol Res 52(12):e8735. https://doi.org/10.1590/1414-431x20198735
Yue XH, Guo L, Wang ZY, Jia TH (2019) Inhibition of miR-17-5p promotes mesenchymal stem cells to repair spinal cord injury. Eur Rev Med Pharmacol Sci 23(9):3899–3907. https://doi.org/10.26355/eurrev_201905_17819
Zhang M, Wang L, Huang S, He X (2020) MicroRNA-223 targets NLRP3 to relieve inflammation and alleviate spinal cord injury. Life Sci 254:117796. https://doi.org/10.1016/j.lfs.2020.117796
Zheng J, Kuang J, Zhang X, Luo D, Liao W (2020) miR-142-3p suppresses apoptosis in spinal cord-injured rats. Transl Neurosci 11(1):105–115. https://doi.org/10.1515/tnsci-2020-0105
Zhou CL, Li F, Wu XW, Cong CL, Liu XD, Tian J, Zheng WZ, Yan JL (2020) Overexpression of miRNA-433-5p protects acute spinal cord injury through activating MAPK1. Eur Rev Med Pharmacol Sci 24(6):2829–2835. https://doi.org/10.26355/eurrev_202003_20644
Funding
This manuscript is not supported by any funds.
Author information
Authors and Affiliations
Contributions
YCS and YW: Conceptualization, YW and HXY: methodology, YW and HXY: investigation, YW: software, YW and HXY: formal analysis, YW and YCS: writing—original draft, YCS: writing—review & editing, YW and YCS: supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest are declared by all of the listed authors.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors approved the submission of this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10571_2022_1235_MOESM5_ESM.pdf
Supplementary file5 (PDF 614 KB) Additional file 5. TSAs of the effect of exosomes on locomotor recovery after SCI. A The adjusted required information size is based on a median value of mean BBB scores of 3.90, an overall significance level (α) of 0.05, a type II risk (β) of 0.1 (power 90%), and equals 423 rats (vertical dotted red line). The cumulative Z-curve (solid blue line) connected by individual studies (small squares) crosses the upper O'Brien–Fleming monitoring boundary of benefit (descending dotted red line). B The adjusted required information size is based on the least value of mean BBB scores of 1, an overall significance level (α) of 0.05, a type II risk (β) of 0.1 (power 90%), and equals 2117 rats (vertical dotted red line). The cumulative Z-curve (solid blue line) connected by individual studies (small squares) crosses the upper O'Brien–Fleming monitoring boundary of benefit (descending dotted red line)
10571_2022_1235_MOESM8_ESM.pdf
Supplementary file8 (PDF 902 KB) Additional file 8. Funnel plot for the strength of pair limbs (A), left fore limbs (B) and right fore limbs (C)
10571_2022_1235_MOESM10_ESM.pdf
Supplementary file10 (PDF 622 KB) Additional file 10. Funnel plot for BBB score of mice at the moment 35th day post-injury
Rights and permissions
About this article
Cite this article
Wang, Y., Yi, H. & Song, Y. miRNA Therapy in Laboratory Models of Acute Spinal Cord Injury in Rodents: A Meta-analysis. Cell Mol Neurobiol 43, 1147–1161 (2023). https://doi.org/10.1007/s10571-022-01235-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-022-01235-2