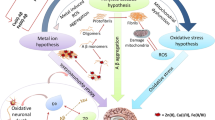
Abstract
While alterations in the locus coeruleus-noradrenergic system are present during early stages of neuropsychiatric disorders, it is unclear what causes these changes and how they contribute to other pathologies in these conditions. Data suggest that the onset of major depressive disorder and schizophrenia is associated with metal dyshomeostasis that causes glial cell mitochondrial dysfunction and hyperactivation in the locus coeruleus. The effect of the overactive locus coeruleus on the hippocampus, amygdala, thalamus, and prefrontal cortex can be responsible for some of the psychiatric symptoms. Although locus coeruleus overactivation may diminish over time, neuroinflammation-induced alterations are presumably ongoing due to continued metal dyshomeostasis and mitochondrial dysfunction. In early Alzheimer’s and Parkinson’s diseases, metal dyshomeostasis and mitochondrial dysfunction likely induce locus coeruleus hyperactivation, pathological tau or α-synuclein formation, and neurodegeneration, while reduction of glymphatic and cerebrospinal fluid flow might be responsible for β-amyloid aggregation in the olfactory regions before the onset of dementia. It is possible that the overactive noradrenergic system stimulates the apoptosis signaling pathway and pathogenic protein formation, leading to further pathological changes which can occur in the presence or absence of locus coeruleus hypoactivation. Data are presented in this review indicating that although locus coeruleus hyperactivation is involved in pathological changes at prodromal and early stages of these neuropsychiatric disorders, metal dyshomeostasis and mitochondrial dysfunction are critical factors in maintaining ongoing neuropathology throughout the course of these conditions. The proposed mechanistic model includes multiple pharmacological sites that may be targeted for the treatment of neuropsychiatric disorders commonly.




Similar content being viewed by others
Data Availability
Not applicable.
References
Abbott NJ, Rönnbäck L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7:41–53. https://doi.org/10.1038/nrn1824
Adams SD, Kouzani AZ, Tye SJ, Bennet KE, Berk M (2018) An investigation into closed-loop treatment of neurological disorders based on sensing mitochondrial dysfunction. J NeuroEng Rehabil 15:8. https://doi.org/10.1186/s12984-018-0349-z
Adani G, Filippini F, Michalke B, Vinceti M (2020) Selenium and other trace elements in the etiology of Parkinson’s disease: a systematic review and meta-analysis of case-control studies. Neuroepidemiology 54:1–23. https://doi.org/10.1159/000502357
Alberdi E, Sánchez-Gómez MV, Cavaliere F, Pérez-Samartín A, Zugaza JL, Trullas R, Domercq M, Matute C (2010) Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium 47:264–272. https://doi.org/10.1016/j.ceca.2009.12.010
Alimonti A, Ristori G, Giubilei F, Stazi MA, Pino A, Visconti A, Brescianini S, Monti MS et al (2007) Serum chemical elements and oxidative status in Alzheimer’s disease, Parkinson disease and multiple sclerosis. Neurotoxicology 28:450–456. https://doi.org/10.1016/j.neuro.2006.12.001
American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders, 5th edn. American Psychiatric Association, Arlington
Andrés-Benito P, Fernández-Dueñas V, Carmona M, Escobar LA, Torrejón-Escribano B, Aso E, Ciruela F, Ferrer I (2017) Locus coeruleus at asymptomatic early and middle Braak stages of neurofibrillary tangle pathology. Neuropathol Appl Neurobiol 43:373–392. https://doi.org/10.1111/nan.12386
Arai Y, Yamazaki M, Mori O, Muramatsu H, Asano G, Katayama Y (2001) α-Synuclein-positive structures in cases with sporadic Alzheimer’s disease: morphology and its relationship to tau aggregation. Brain Res 888:287–296. https://doi.org/10.1016/s0006-8993(00)03082-1
Arnal N, Cristalli DO, de Alaniz MJT, Marra CA (2010) Clinical utility of copper, ceruloplasmin, and metallothionein plasma determinations in human neurodegenerative patients and their first-degree relatives. Brain Res 1319:118–130. https://doi.org/10.1016/j.brainres.2009.11.085
Arnsten AFT, Raskind MA, Taylor FB, Connor DF (2015) The effects of stress exposure on prefrontal cortex: translating basic research into successful treatments for post-traumatic stress disorder. Neurobiol Stress 1:89–99. https://doi.org/10.1016/j.ynstr.2014.10.002
Aston-Jones GS, Iba M, Clayton E, Rajkowski J, Cohen J (2007) The locus coeruleus and regulation of behavioral flexibility and attention: clinical implications. In: Ordway GA, Schwartz MA, Frazer A (eds) Brain norepinephrine: neurobiology and therapeutics. Cambridge University Press, Cambridge, pp 169–235
Baglioni C, Spiegelhalder K, Regen W, Feige B, Nissen C, Lombardo C, Violani C, Hennig J et al (2014) Insomnia disorder is associated with increased amygdala reactivity to insomnia-related stimuli. Sleep 37:1907–1917. https://doi.org/10.5665/sleep.4240
Bansal Y, Kuhad A (2016) Mitochondrial dysfunction in depression. Curr Neuropharmacol 14:610–618. https://doi.org/10.2174/1570159x14666160229114755
Bari BA, Chokshi V, Schmidt K (2020) Locus coeruleus-norepinephrine: basic functions and insights into Parkinson’s disease. Neural Regen Res 15:1006–1013. https://doi.org/10.4103/1673-5374.270297
Barnham KJ, Bush AI (2014) Biological metals and metal-targeting compounds in major neurodegenerative diseases. Chem Soc Rev 43:6727. https://doi.org/10.1039/c4cs00138a
Baskey G, Singh A, Sharma R, Mallick BN (2009) REM sleep deprivation-induced noradrenaline stimulates neuronal and inhibits glial Na-K ATPase in rat brain: in vivo and in vitro studies. Neurochem Int 54:65–71. https://doi.org/10.1016/j.neuint.2008.10.006
Baum L, Chan IHS, Cheung SK-K, Goggins WB, Mok V, Lam L, Leung V, Hui E et al (2010) Serum zinc is decreased in Alzheimer’s disease and serum arsenic correlates positively with cognitive ability. Biometals 23:173–179. https://doi.org/10.1007/s10534-009-9277-5
Bellesi M, de Vivo L, Chini M, Gilli F, Tononi G, Cirelli C (2017) Sleep loss promotes astrocytic phagocytosis and microglial activation in mouse cerebral cortex. J Neurosci 37:5263–5273. https://doi.org/10.1523/JNEUROSCI.3981-16.2017
Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS et al (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38:515–517. https://doi.org/10.1038/ng1769
Betts MJ, Kirilina E, Otaduy MCG, Ivanov D, Acosta-Cabronero J, Callaghan MF, Lambert C, Cardenas-Blanco A et al (2019) Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain 142:2558–2571. https://doi.org/10.1093/brain/awz193
Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, de Clercq E, Vescovi A, Bagetta G et al (2001) CXCR4-activated astrocyte glutamate release via TNFα: amplification by microglia triggers neurotoxicity. Nat Neurosci 4:702–710. https://doi.org/10.1038/89490
Bieri G, Gitler AD, Brahic M (2018) Internalization, axonal transport and release of fibrillar forms of alpha-synuclein. Neurobiol Dis 109:219–225. https://doi.org/10.1016/j.nbd.2017.03.007
Bin Y, Li X, He Y, Chen S, Xiang J (2013) Amyloid-β peptide (1–42) aggregation induced by copper ions under acidic conditions. Acta Biochim Biophys Sin 45:570–577. https://doi.org/10.1093/abbs/gmt044
Bonkowski D, Katyshev V, Balabanov RD, Borisov A, Dore-Duffy P (2011) The CNS microvascular pericyte: pericyte-astrocyte crosstalk in the regulation of tissue survival. Fluids Barriers CNS 8:8. https://doi.org/10.1186/2045-8118-8-8
Bornhorst J, Wehe CA, Hüwel S, Karst U, Galla H-J, Schwerdtle T (2012) Impact of manganese on and transfer across blood-brain and blood-cerebrospinal fluid barrier in vitro. J Biol Chem 287:17140–17151. https://doi.org/10.1074/jbc.M112.344093
Braak H, Thal DR, Ghebremedhin E, Tredici KD (2011) Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. J Neuropathol Exp Neurol 70:960–969. https://doi.org/10.1097/NEN.0b013e318232a379
Brewer GJ, Kaur S (2013) Zinc deficiency and zinc therapy efficacy with reduction of serum free copper in Alzheimer’s disease. Int J Alzheimers Dis 2013:586365. https://doi.org/10.1155/2013/586365
Brown DR (2013) α-Synuclein as a ferrireductase. Biochem Soc Trans 41:1513–1517. https://doi.org/10.1042/BST20130130
Brown RE, Basheer R, McKenna JT, Strecker RE, McCarley RW (2012) Control of sleep and wakefulness. Physiol Rev 92:1087–1187. https://doi.org/10.1152/physrev.00032.2011
Brown SSG, Rutland JW, Verma G, Feldman RE, Alper J, Schneider M, Delman BN, Murrough JM et al (2019) Structural MRI at 7T reveals amygdala nuclei and hippocampal subfield volumetric association with major depressive disorder symptom severity. Sci Rep 9:10166. https://doi.org/10.1038/s41598-019-46687-7
Bruno A, Zoccali RA, Abenavoli E, Pandolfo G, Scimeca G, Spina E, Muscatello MRA (2014) Augmentation of clozapine with agomelatine in partial-responder schizophrenia: a 16-week, open-label, uncontrolled pilot study. J Clin Psychopharmacol 34:491–494. https://doi.org/10.1097/JCP.0000000000000157
Bucossi S, Polimanti R, Mariani S, Ventriglia M, Bonvicini C, Migliore S, Manfellotto D, Salustri C et al (2012) Association of K832R and R952K SNPs of Wilson’s disease gene with Alzheimer’s disease. J Alzheimers Dis 29:913–919. https://doi.org/10.3233/JAD-2012-111997
Busche MA, Hyman BT (2020) Synergy between amyloid-β and tau in Alzheimer’s disease. Nat Neurosci 23:1183–1193. https://doi.org/10.1038/s41593-020-0687-6
Cardinali DP, Vigo DE, Olivar N, Vidal MF, Furio AM, Brusco LI (2012) Therapeutic application of melatonin in mild cognitive impairment. Am J Neurodegener Dis 1:280–291
Chalbot S, Zetterberg H, Blennow K, Fladby T, Andreasen N, Grundke-Iqbal I, Iqbal K (2011) Blood-cerebrospinal fluid barrier permeability in Alzheimer’s disease. J Alzheimers Dis 25:505–515. https://doi.org/10.3233/JAD-2011-101959
Chan RCK, Di X, McAlonan GM, Gong Q (2011) Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull 37:177–188. https://doi.org/10.1093/schbul/sbp073
Chandra A, Farrell C, Wilson H, Dervenoulas G, De Natale ER, Politis M (2021) Alzheimer’s Disease Neuroimaging Initiative., Aquaporin-4 polymorphisms predict amyloid burden and clinical outcome in the Alzheimer’s disease spectrum. Neurobiol Aging 97:1–9. https://doi.org/10.1016/j.neurobiolaging.2020.06.007
Chen HB, Chan Y-T, Hung AC, Tsai Y-C, Sun SH (2006) Elucidation of ATP stimulated stress protein expression of RBA-2 type-2 astrocytes: ATP potentiate HSP60 and Cu/Zn SOD expression and stimulates pI shift of peroxiredoxin II. J Cell Biochem 97:314–326. https://doi.org/10.1002/jcb.20547
Cheng F, Li X, Li Y, Wang C, Wang T, Liu G, Baskys A, Uéda K et al (2011) α-Synuclein promotes clathrin-mediated NMDA receptor endocytosis and attenuates NMDA-induced dopaminergic cell death. J Neurochem 119:815–825. https://doi.org/10.1111/j.1471-4159.2011.07460.x
Christen-Zaech S, Kraftsik R, Pillevuit O, Kiraly M, Martins R, Khalili K, Miklossy J (2003) Early olfactory involvement in Alzheimer’s disease. Can J Neurol Sci 30:20–25. https://doi.org/10.1017/s0317167100002389
Cicero CE, Mostile G, Vasta R, Rapisarda V, Signorelli SS, Ferrante M, Zappia M, Nicoletti A (2017) Metals and neurodegenerative diseases. A systematic review. Environ Res 159:82–94. https://doi.org/10.1016/j.envres.2017.07.048
Clarke LE, Liddelow SA, Chakraborty C, Münch AE, Heiman M, Barres BA (2018) Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci USA 115:E1896–E1905. https://doi.org/10.1073/pnas.1800165115
Członkowska A, Litwin T, Dusek P, Ferenci P, Lutsenko S, Medici V, Rybakowski JK, Weiss KH et al (2018) Wilson Disease Nat Rev Dis Primers 4:21. https://doi.org/10.1038/s41572-018-0018-3
Dasari AKR, Kayed R, Wi S, Lim KH (2019) Tau interacts with the C-terminal region of α-synuclein, promoting formation of toxic aggregates with distinct molecular conformations. Biochemistry 58:2814–2821. https://doi.org/10.1021/acs.biochem.9b00215
De Marco G, Lomartire A, Mandili G, Lupino E, Buccinnà B, Ramondetti C, Moglia C, Novelli F et al (2014) Reduced cellular Ca2+ availability enhances TDP-43 cleavage by apoptotic caspases. Biochim Biophys Acta 1843:725–734. https://doi.org/10.1016/j.bbamcr.2014.01.010
Demarin V, Podobnik SS, Storga-Tomic D, Kay G (2004) Treatment of Alzheimer’s disease with stabilized oral nicotinamide adenine dinucleotide: a randomized, double-blind study. Drugs Exp Clin Res 30:27–33
Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Lees AJ, Jenner P et al (1991) Alterations in the levels of iron, ferritin and other trace metals in Parkinson’s disease and other neurodegenerative diseases affecting the basal ganglia. Brain 114(4):1953–1975. https://doi.org/10.1093/brain/114.4.1953
Dimitrov S, Besedovsky L, Born J, Lange T (2015) Differential acute effects of sleep on spontaneous and stimulated production of tumor necrosis factor in men. Brain Behav Immun 47:201–210. https://doi.org/10.1016/j.bbi.2014.11.017
Diógenes MJ, Dias RB, Rombo DM, Vicente Miranda H, Maiolino F, Guerreiro P, Näsström T, Franquelim HG et al (2012) Extracellular α-synuclein oligomers modulate synaptic transmission and impair LTP via NMDA-receptor activation. J Neurosci 32:11750–11762. https://doi.org/10.1523/JNEUROSCI.0234-12.2012
Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL (2010) A meta-analysis of cytokines in major depression. Biol Psychiatry 67:446–457. https://doi.org/10.1016/j.biopsych.2009.09.033
Dowling GA, Mastick J, Colling E, Carter JH, Singer CM, Aminoff MJ (2005) Melatonin for sleep disturbances in Parkinson’s disease. Sleep Med 6:459–466. https://doi.org/10.1016/j.sleep.2005.04.004
Du K, Liu M, Pan Y, Zhong X, Wei M (2017) Association of serum manganese levels with Alzheimer’s disease and mild cognitive impairment: a systematic review and meta-analysis. Nutrients 29:231. https://doi.org/10.3390/nu9030231
Dusek P, Litwin T, Członkowska A (2019) Neurologic impairment in Wilson disease. Ann Transl Med 7(Suppl 2):S64. https://doi.org/10.21037/atm.2019.02.43
Dusek P, Roos PM, Litwin T, Schneider SA, Flaten TP, Aaseth J (2015) The neurotoxicity of iron, copper and manganese in Parkinson’s and Wilson’s diseases. J Trace Elem Med Biol 31:193–203. https://doi.org/10.1016/j.jtemb.2014.05.007
Eban-Rothschild A, Appelbaum L, de Lecea L (2018) Neuronal mechanisms for sleep/wake regulation and modulatory drive. Neuropsychopharmacology 43:937–952. https://doi.org/10.1038/npp.2017.294
Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K (2010) Cell-produced α-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 30:6838–6851. https://doi.org/10.1523/JNEUROSCI.5699-09.2010
Engelhardt B, Sorokin L (2009) The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol 31:497–511. https://doi.org/10.1007/s00281-009-0177-0
Esposito L, Raber J, Kekonius L, Yan F, Yu GQ, Bien-Ly N, Puoliväli J, Scearce-Levie K et al (2006) Reduction in mitochondrial superoxide dismutase modulates Alzheimer’s disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. J Neurosci 26:5167–5179. https://doi.org/10.1523/JNEUROSCI.0482-06.2006
Faux NG, Ritchie CW, Gunn A, Rembach A, Tsatsanis A, Bedo J, Harrison J, Lannfelt L et al (2010) PBT2 rapidly improves cognition in Alzheimer’s Disease: additional phase II analyses. J Alzheimers Dis 20:509–516. https://doi.org/10.3233/JAD-2010-1390
Ferger AI, Campanelli L, Reimer V, Muth KN, Merdian I, Ludolph AC, Witting A (2010) Effects of mitochondrial dysfunction on the immunological properties of microglia. J Neuroinflamm 7:45. https://doi.org/10.1186/1742-2094-7-45
Fischer LR, Igoudjil A, Magrané J, Li Y, Hansen JM, Manfredi G, Glass JD (2011) SOD1 targeted to the mitochondrial intermembrane space prevents motor neuropathy in the Sod1 knockout mouse. Brain 134(1):196–209. https://doi.org/10.1093/brain/awq314
Fleischman D, Berdahl JP, Zaydlarova J, Stinnett S, Fautsch MP, Allingham RR (2012) Cerebrospinal fluid pressure decreases with older age. PLoS ONE 7:e52664. https://doi.org/10.1371/journal.pone.0052664
Foote SL, Bloom FE, Aston-Jones G (1983) Nucleus locus coeruleus: new evidence of anatomical and physiological specificity. Physiol Rev 63:844–914. https://doi.org/10.1152/physrev.1983.63.3.844
Gahr M (2014) Agomelatine in the treatment of major depressive disorder: an assessment of benefits and risks. Curr Neuropharmacol 12:287–398. https://doi.org/10.2174/1570159X12999140619122914
Ganesh Yerra V, Negi G, Sharma SS, Kumar A (2013) Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-κB pathways in diabetic neuropathy. Redox Biol 1:394–397. https://doi.org/10.1016/j.redox.2013.07.005
García-Lorenzo D, Santos CL, Ewenczyk C, Leu-Semenescu S, Gallea C, Quattrocchi G, Lobo PP, Poupon C et al (2013) The coeruleus/subcoeruleus complex in rapid eye movement sleep behaviour disorders in Parkinson’s disease. Brain 136:2120–2129. https://doi.org/10.1093/brain/awt152
Garver DL, Tamas RL, Holcomb JA (2003) Elevated interleukin-6 in the cerebrospinal fluid of a previously delineated schizophrenia subtype. Neuropsychopharmacology 28:1515–1520. https://doi.org/10.1038/sj.npp.1300217
Gholipour Baradari A, Alipour A, Mahdavi A, Sharifi H, Nouraei SM, Emami Zeydi A (2018) The effect of zinc supplementation on sleep quality of ICU nurses: a double blinded randomized controlled trial. Workplace Health Saf 66:191–200. https://doi.org/10.1177/2165079917734880
Goedert M (2015) Neurodegeneration. Alzheimer’s and Parkinson’s diseases: the prion concept in relation to assembled Aβ, tau, and α-synuclein. Science 349:1255555. https://doi.org/10.1126/science.1255555
Grieve SM, Korgaonkar MS, Koslow SH, Gordon E, Williams LM (2013) Widespread reductions in gray matter volume in depression. Neuroimage Clin 3:332–339. https://doi.org/10.1016/j.nicl.2013.08.016
Gromadzka G, Tarnacka B, Flaga A, Adamczyk A (2020) Copper dyshomeostasis in neurodegenerative diseases-therapeutic implications. Int J Mol Sci 21:9259. https://doi.org/10.3390/ijms21239259
Grønli O, Kvamme JM, Friborg O, Wynn R (2013) Zinc deficiency is common in several psychiatric disorders. PLoS ONE 8:e82793. https://doi.org/10.1371/journal.pone.0082793
Gustafsson G, Lööv C, Persson E, Lázaro DF, Takeda S, Bergström J, Erlandsson A, Sehlin D et al (2018) Secretion and uptake of α-synuclein via extracellular vesicles in cultured cells. Cell Mol Neurobiol 38:1539–1550. https://doi.org/10.1007/s10571-018-0622-5
Ha C, Ryu J, Park CB (2007) Metal ions differentially influence the aggregation and deposition of Alzheimer’s β-amyloid on a solid template. Biochemistry 46:6118–6125. https://doi.org/10.1021/bi7000032
Hackett TA (2015) Anatomic organization of the auditory cortex. Handb Clin Neurol 129:27–53. https://doi.org/10.1016/B978-0-444-62630-1.00002-0
Hall LM, Klimes-Dougan B, Hunt RH, Thomas KM, Houri A, Noack E, Mueller BA, Lim KO et al (2014) An fMRI study of emotional face processing in adolescent major depression. J Affect Disord 168:44–50. https://doi.org/10.1016/j.jad.2014.06.037
Halliday GM, McCann H (2010) The progression of pathology in Parkinson’s disease. Ann NY Acad Sci 1184:188–195. https://doi.org/10.1111/j.1749-6632.2009.05118.x
Hara T, Takeda TA, Takagishi T, Fukue K, Kambe T, Fukada T (2017) Physiological roles of zinc transporters: molecular and genetic importance in zinc homeostasis. J Physiol Sci 67:283–301. https://doi.org/10.1007/s12576-017-0521-4
Heatherton TF, Wagner DD (2011) Cognitive neuroscience of self-regulation failure. Trends Cogn Sci 15:132–139. https://doi.org/10.1016/j.tics.2010.12.005
Hegde ML, Shanmugavelu P, Vengamma B, Rao TSS, Menon RB, Rao RV, Rao KSJ (2004) Serum trace element levels and the complexity of inter-element relations in patients with Parkinson’s disease. J Trace Elem Med Biol 18:163–171. https://doi.org/10.1016/j.jtemb.2004.09.003
Hertenstein E, Feige B, Gmeiner T, Kienzler C, Spiegelhalder K, Johann A, Jansson-Fröjmark M, Palagini L et al (2019) Insomnia as a predictor of mental disorders: a systematic review and meta-analysis. Sleep Med Rev 43:96–105. https://doi.org/10.1016/j.smrv.2018.10.006
Hesketh S, Sassoon J, Knight R, Brown DR (2008) Elevated manganese levels in blood and CNS in human prion disease. Mol Cell Neurosci 37:590–598. https://doi.org/10.1016/j.mcn.2007.12.008
Hirschtritt ME, Olfson M, Kroenke K (2021) Balancing the risks and benefits of benzodiazepines. JAMA 325:347–348. https://doi.org/10.1001/jama.2020.22106
Holth JK, Fritschi SK, Wang C, Pedersen NP, Cirrito JR, Mahan TE, Finn MB, Manis M et al (2019) The sleep-wake cycle regulates brain interstitial fluid tau in mice and CSF tau in humans. Science 363:880–884. https://doi.org/10.1126/science.aav2546
Horn D, Barrientos A (2008) Mitochondrial copper metabolism and delivery to cytochrome c oxidase. IUBMB Life 60:421–429. https://doi.org/10.1002/iub.50
Horowitz MP, Greenamyre JT (2010) Mitochondrial iron metabolism and its role in neurodegeneration. J Alzheimers Dis 20(2):551–568. https://doi.org/10.3233/JAD-2010-100354
Huang M-L, Khoh T-T, Lu S-J, Pan F, Chen J-K, Hu J-B, Hu S-H, Xu W-J et al (2017) Relationships between dorsolateral prefrontal cortex metabolic change and cognitive impairment in first-episode neuroleptic-naive schizophrenia patients. Medicine 96:e7228. https://doi.org/10.1097/MD.0000000000007228
Ichikawa N, Lisi G, Yahata N, Okada G, Takamura M, Yamada HRI, T, Yamada M, et al (2020) Primary functional brain connections associated with melancholic major depressive disorder and modulation by antidepressants. Sci Rep 10:3542. https://doi.org/10.1038/s41598-020-60527-z
Ignatenko O, Chilov D, Paetau I, de Miguel E, Jackson CB, Capin G, Paetau A, Terzioglu M et al (2018) Loss of mtDNA activates astrocytes and leads to spongiotic encephalopathy. Nat Commun 9:70. https://doi.org/10.1038/s41467-017-01859-9
Ihalainen JA, Tanila H (2002) In vivo regulation of dopamine and noradrenaline release by α2A-adrenoceptors in the mouse prefrontal cortex. Eur J Neurosci 15:1789–1794. https://doi.org/10.1046/j.1460-9568.2002.02014.x
Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE et al (2012) A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4:147ra111. https://doi.org/10.1126/scitranslmed.3003748
Ingrassia R, Garavaglia B, Memo M (2019) DMT1 expression and iron levels at the crossroads between aging and neurodegeneration. Front Neurosci 13:575. https://doi.org/10.3389/fnins.2019.00575
Iseki E, Togo T, Suzuki K, Katsuse O, Marui W, de Silva R, Lees A, Yamamoto T et al (2003) Dementia with Lewy bodies from the perspective of tauopathy. Acta Neuropathol 105:265–270. https://doi.org/10.1007/s00401-002-0644-3
Ishii Y, Yamaizumi A, Kawakami A, Islam A, Choudhury ME, Takahashi H, Yano H, Tanaka J (2015) Anti-inflammatory effects of noradrenaline on LPS-treated microglial cells: suppression of NFκB nuclear translocation and subsequent STAT1 phosphorylation. Neurochem Int 90:56–66. https://doi.org/10.1016/j.neuint.2015.07.010
Islam MR, Islam MR, Qusar MMAS, Islam MS, Kabir MH, Rahman GKMM, Islam MS, Hasnat A (2018) Alterations of serum macro-minerals and trace elements are associated with major depressive disorder: a case-control study. BMC Psychiatry 18:94. https://doi.org/10.1186/s12888-018-1685-z
Jack CR Jr, Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, Shaw LM, Vemuri P et al (2013) Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol 12:207–216. https://doi.org/10.1016/S1474-4422(12)70291-0
Jack CR Jr, Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ (2010) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9:119–128. https://doi.org/10.1016/S1474-4422(09)70299-6
James SA, Volitakis I, Adlard PA, Duce JA, Masters CL, Cherny RA, Bush AI (2012) Elevated labile Cu is associated with oxidative pathology in Alzheimer disease. Free Radic Biol Med 52:298–302. https://doi.org/10.1016/j.freeradbiomed.2011.10.446
Jamwal S, Blackburn JK, Elsworth JD (2021) PPARγ/PGC1α signaling as a potential therapeutic target for mitochondrial biogenesis in neurodegenerative disorders. Pharmacol Ther 219:107705. https://doi.org/10.1016/j.pharmthera.2020.107705
Jansen WJ, Ossenkoppele R, Knol DL, Tijms BM, Scheltens P, Verhey FRJ, Visser PJ (2015) Prevalence of cerebral amyloid pathology in persons without dementia: a meta-analysis. JAMA 313:1924–1938. https://doi.org/10.1001/jama.2015.4668
Jiménez-Jiménez FJ, Molina JA, Aguilar MV, Meseguer I, Mateos-Vega CJ, González-Muñoz MJ, de Bustos F, Martínez-Salio A et al (1998) Cerebrospinal fluid levels of transition metals in patients with Parkinson’s disease. J Neural Transm 105:497–505. https://doi.org/10.1007/s007020050073
Joo EY, Noh HJ, Kim J-S, Koo DL, Kim D, Hwang KJ, Kim J, Kim ST et al (2013) Brain gray matter deficits in patients with chronic primary insomnia. Sleep 36:999–1007. https://doi.org/10.5665/sleep.2796
Jucker M, Walker LC (2013) Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature 501:45–51. https://doi.org/10.1038/nature12481
Kagota S, Morikawa K, Ishida H, Chimoto J, Maruyama-Fumoto K, Yamada S, Shinozuka K (2021) Vasorelaxant effects of benzodiazepines, non-benzodiazepine sedative-hypnotics, and tandospirone on isolated rat arteries. Eur J Pharmacol 892:173744. https://doi.org/10.1016/j.ejphar.2020.173744
Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P, Lepack A, Majik MS et al (2012) Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med 18:1413–1417. https://doi.org/10.1038/nm.2886
Kaskie RE, Graziano B, Ferrarelli F (2017) Schizophrenia and sleep disorders: links, risks, and management challenges. Nat Sci Sleep 9:227–239. https://doi.org/10.2147/NSS.S121076
Katsuoka F, Yamamoto M (2016) Small Maf proteins (MafF, MafG, MafK): history, structure and function. Gene 586:197–205. https://doi.org/10.1016/j.gene.2016.03.058
Kawada R, Yoshizumi M, Hirao K, Fujiwara H, Miyata J, Shimizu M, Namiki C, Sawamoto N et al (2009) Brain volume and dysexecutive behavior in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 33:1255–1260. https://doi.org/10.1016/j.pnpbp.2009.07.014
Kenney PM, Bennett JP Jr (2019) Alzheimer’s disease frontal cortex mitochondria show a loss of individual respiratory proteins but preservation of respiratory supercomplexes. Int J Alzheimers Dis 2019:4814783. https://doi.org/10.1155/2019/4814783
Kennedy SH, Emsley R (2006) Placebo-controlled trial of agomelatine in the treatment of major depressive disorder. Eur Neuropsychopharmacol 16:93–100. https://doi.org/10.1016/j.euroneuro.2005.09.002
Kettenmann H, Hanisch UK, Noda M, Verhratskym A (2011) Physiology of microglia. Physiol Rev 91:461–553. https://doi.org/10.1152/physrev.00011.2010
Kiernan JA (2012) Anatomy of the temporal lobe. Epilepsy Res Treat 2012:176157. https://doi.org/10.1155/2012/176157
Kim I, Park EJ, Seo J, Ko SJ, Lee J, Kim CH (2011) Zinc stimulates tau S214 phosphorylation by the activation of Raf/mitogen-activated protein kinase-kinase/extracellular signal-regulated kinase pathway. NeuroReport 22:839–844
Kitzberger R, Madl C, Ferenci P (2005) Wilson disease. Metab Brain Dis 20:295–302. https://doi.org/10.1007/s11011-005-7910-8
Klimek V, Stockmeier C, Overholser J, Meltzer HY, Kalka S, Dilley G, Ordway GA (1997) Reduced levels of norepinephrine transporters in the locus coeruleus in major depression. J Neurosci 17:8451–8458. https://doi.org/10.1523/JNEUROSCI.17-21-08451.1997
Koh JY, Lee SJ (2020) Metallothionein-3 as a multifunctional player in the control of cellular processes and diseases. Mol Brain 13:116. https://doi.org/10.1186/s13041-020-00654-w
Kohtala S, Alitalo O, Rosenholm M, Rozov S, Rantamäki T (2021) Time is of the essence: coupling sleep-wake and circadian neurobiology to the antidepressant effects of ketamine. Pharmacol Ther 221:107741. https://doi.org/10.1016/j.pharmthera.2020.107741
Kornhuber J, Lange KW, Kruzik P, Rausch WD, Gabriel E, Jellinger K, Riederer P (1994) Iron, copper, zinc, magnesium, and calcium in postmortem brain tissue from schizophrenic patients. Biol Psychiatry 36:31–34. https://doi.org/10.1016/0006-3223(94)90059-0
Lannfelt L, Blennow K, Zetterberg H, Batsman S, Ames D, Harrison J, Masters CL, Targum S et al (2008) Safety, efficacy, and biomarker findings of PBT2 in targeting Aβ as a modifying therapy for Alzheimer’s disease: a phase IIa, double-blind, randomised, placebo controlled trial. Lancet Neurol 7:779–786. https://doi.org/10.1016/S1474-4422(08)70167-4
Lashuel HA, Overk CR, Oueslati A, Masliah E (2013) The many faces of α-synuclein: from structure and toxicity to therapeutic target. Nat Rev Neurosci 14:38–48. https://doi.org/10.1038/nrn3406
Leng Y, Musiek ES, Hu K, Cappuccio FP, Yaffe K (2019) Association between circadian rhythms and neurodegenerative diseases. Lancet Neurol 18:307–318. https://doi.org/10.1016/S1474-4422(18)30461-7
Li GJ, Zheng W (2005) Regulation of neuroactivemetals by the choroid plexus. In: Zheng W, Chodobski A (eds) The blood-cerebrospinal fluid barrier. CRC Press, New York, pp 211–239
Li M, Yan J, Li S, Wang T, Wen H, Yin Y, Fu S, Zeng L et al (2018) Altered gray matter volume in primary insomnia patients: a DARTEL-VBM study. Brain Imaging Behav 12:1759–1767. https://doi.org/10.1007/s11682-018-9844-x
Li L, Yang X (2018) The essential element manganese, oxidative stress, and metabolic diseases: links and interactions. Oxid Med Cell Longev 2018:7580707. https://doi.org/10.1155/2018/7580707
Liang Z, Currais A, Soriano-Castell D, Schubert D, Maher P (2021) Natural products targeting mitochondria: emerging therapeutics for age-associated neurological disorders. Pharmacol Ther 221:107749. https://doi.org/10.1016/j.pharmthera.2020.107749
Lilius TO, Blomqvist K, Hauglund NL, Liu G, Stæger FF, Bærentzen S, Du T, Ahlström F et al (2019) Dexmedetomidine enhances glymphatic brain delivery of intrathecally administered drugs. J Control Release 304:29–38. https://doi.org/10.1016/j.jconrel.2019.05.005
Lim MM, Gerstner JR, Holtzman DM (2014) The sleep-wake cycle and Alzheimer’s disease: what do we know? Neurodegener Dis Manag 4:351–362. https://doi.org/10.2217/nmt.14.33
Litwin T, Dusek P, Szafrański T, Dzieżyc K, Członkowska A, Rybakowski JK (2018) Psychiatric manifestations in Wilson’s disease: possibilities and difficulties for treatment. Ther Adv Psychopharmacol 8:199–211. https://doi.org/10.1177/2045125318759461
Liu X, Zhong S, Li Z, Chen J, Wang Y, Lai S, Miao H, Jia Y (2020) Serum copper and zinc levels correlate with biochemical metabolite ratios in the prefrontal cortex and lentiform nucleus of patients with major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry 99:109828. https://doi.org/10.1016/j.pnpbp.2019.109828
Liu D-X, He X, Wu D, Zhang Q, Yang C, Liang F-Y, He X-F, Dai G-Y et al (2017) Continuous theta burst stimulation facilitates the clearance efficiency of the glymphatic pathway in a mouse model of sleep deprivation. Neurosci Lett 653:189–194. https://doi.org/10.1016/j.neulet.2017.05.064
Liu T, Lu Q-B, Yan L, Guo J, Feng F, Qiu J, Wang J (2015) Comparative study on serum levels of 10 trace elements in schizophrenia. PLoS ONE 10:e0133622. https://doi.org/10.1371/journal.pone.0133622
Liu YU, Ying Y, Li Y, Eyo UB, Chen T, Zheng J, Umpierre AD, Zhu J et al (2019) Neuronal network activity controls microglial process surveillance in awake mice via norepinephrine signaling. Nat Neurosci 22:1771–1781. https://doi.org/10.1038/s41593-019-0511-3
Loddo G, Calandra-Buonaura G, Sambati L, Giannini G, Cecere A, Cortelli P, Provini F (2017) The treatment of sleep disorders in Parkinson’s disease: from research to clinical practice. Front Neurol 8:42. https://doi.org/10.3389/fneur.2017.00042
Louveau A, Harris TH, Kipnis J (2015) Revisiting the mechanisms of CNS immune privilege. Trends Immunol 36:569–577. https://doi.org/10.1016/j.it.2015.08.006
Lu J, Zhang S, Ma X, Jia C, Liu Z, Huang C, Liu C, Li D (2020) Structural basis of the interplay between α-synuclein and tau in regulating pathological amyloid aggregation. J Biol Chem 295:7470–7480. https://doi.org/10.1074/jbc.RA119.012284
Maes M, De Vos N, Demedts P, Wauters A, Neels H (1999) Lower serum zinc in major depression in relation to changes in serum acute phase proteins. J Affect Disord 56:189–194. https://doi.org/10.1016/s0165-0327(99)00011-7
Maes M, Vandoolaeghe E, Neels H, Demedts P, Wauters A, Meltzer HY, Altamura C, Desnyder R (1997) Lower serum zinc in major depression is a sensitive marker of treatment resistance and of the immune/inflammatory response in that illness. Biol Psychiatry 42:349–358. https://doi.org/10.1016/S0006-3223(96)00365-4
Martin-Bastida A, Ward RJ, Newbould R, Piccini P, Sharp D, Kabba C, Patel MC, Spino M et al (2017) Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson’s disease. Sci Rep 7:1398–1399. https://doi.org/10.1038/s41598-017-01402-2
Martín-Hernández D, Caso JR, Meana JJ, Callado LF, Madrigal JLM, García-Bueno B, Leza JC (2018) Intracellular inflammatory and antioxidant pathways in postmortem frontal cortex of subjects with major depression: effect of antidepressants. J Neuroinflammation 15:251. https://doi.org/10.1186/s12974-018-1294-2
Medeiros CA, Carvalhedo de Bruin PF, Lopes LA, Magalhães MC, de Lourdes SM, de Bruin VM (2007) Effect of exogenous melatonin on sleep and motor dysfunction in Parkinson’s disease: a randomized, double blind, placebo-controlled study. J Neurol 254:459–464. https://doi.org/10.1007/s00415-006-0390-x
Mendelson N, Gontmacher B, Vodonos A, Novack V, Abu-AjAj M, Wolak A, Shalev H, Wolak T (2018) Benzodiazepine consumption is associated with lower blood pressure in ambulatory blood pressure monitoring (ABPM): retrospective analysis of 4938 ABPMs. Am J Hypertens 31:431–437. https://doi.org/10.1093/ajh/hpx188
Mestre H, Mori Y, Nedergaard M (2020) The brain’s glymphatic system: current controversies. Trends Neurosci 43:458–466. https://doi.org/10.1016/j.tins.2020.04.003
Milardi D, Quartarone A, Bramanti A, Anastasi G, Bertino S, Basile GA, Buonasera P, Pilone G et al (2019) The cortico-basal ganglia-cerebellar network: past, present and future perspectives. Front Syst Neurosci 13:61. https://doi.org/10.3389/fnsys.2019.00061
Mo Z-Y, Zhu Y-Z, Zhu H-L, Fan J-B, Chen J, Liang Y (2009) Low micromolar zinc accelerates the fibrillization of human tau via bridging of Cys-291 and Cys-322. J Biol Chem 284:34648–34657. https://doi.org/10.1074/jbc.M109.058883
Molina JA, Jiménez-Jiménez FJ, Aguilar MV, Meseguer I, Mateos-Vega CJ, González-Muñoz MJ, de Bustos F, Porta J et al (1998) Cerebrospinal fluid levels of transition metals in patients with Alzheimer’s disease. J Neural Transm 105:479–488. https://doi.org/10.1007/s007020050071
Moreira GG, Cristóvão JS, Torres VM, Carapeto AP, Rodrigues MS, Landrieu I, Cordeiro C, Gomes CM (2019) Zinc binding to tau influences aggregation kinetics and oligomer distribution. Int J Mol Sci 20:5979. https://doi.org/10.3390/ijms20235979
Morris G, Puri BK, Maes M, Olive L, Berk M, Carvalho AF (2020) The role of microglia in neuroprogressive disorders: mechanisms and possible neurotherapeutic effects of induced ketosis. Prog Neuropsychopharmacol Biol Psychiatry 99:109858. https://doi.org/10.1016/j.pnpbp.2020.109858
Mortazavi M, Farzin D, Zarhghami M, Hosseini SH, Mansoori P, Nateghi G (2015) Efficacy of zinc sulfate as an add-on therapy to risperidone versus risperidone alone in patients with schizophrenia: a double-blind randomized placebo-controlled trial. Iran J Psychiatry Behav Sci 9:e853. https://doi.org/10.17795/ijpbs-853
Murphy M, Peterson MJ (2015) Sleep Disturbances in Depression. Sleep Med Clin 10:17–23. https://doi.org/10.1016/j.jsmc.2014.11.009
Murru S, Hess S, Barth E, Almajan ER, Schatton D, Hermans S, Brodesser S, Lange T et al (2019) Astrocyte-specific deletion of the mitochondrial m-AAA protease reveals glial contribution to neurodegeneration. Glia 67:1526–1541. https://doi.org/10.1002/glia.23626
Nakada T, Kwee IL (2019) Fluid dynamics inside the brain barrier: current concept of interstitial flow, glymphatic flow, and cerebrospinal fluid circulation in the brain. Neuroscientist 25:155–166. https://doi.org/10.1177/1073858418775027
Nakagawa Y (2017) Psycho-behavioral spiral of disturbances in prosocial behavior, stress response, and self-regulation in substance-related and addictive disorders. J Drug Alcohol Res 6:236017. https://doi.org/10.4303/jdar/236017
Nakagawa Y, Chiba K (2014) Role of microglial M1/M2 polarization in relapse and remission of psychiatric disorders and diseases. Pharmaceuticals 7:1028–1048. https://doi.org/10.3390/ph7121028
Nakagawa Y, Chiba K (2015) Diversity and plasticity of microglial cells in psychiatric and neurological disorders. Pharmacol Ther 154:21–35. https://doi.org/10.1016/j.pharmthera.2015.06.010
Nakagawa Y, Chiba K (2016) Involvement of neuroinflammation during brain development in social cognitive deficits in autism spectrum disorder and schizophrenia. J Pharmacol Exp Therapeut 358:504–515. https://doi.org/10.1124/jpet.116.234476
Nakagawa Y, Yamada S (2020) Metal homeostasis disturbances in neurodegenerative disorders, with special emphasis on Creutzfeldt-Jakob disease – potential pathogenetic mechanism and therapeutic implications. Pharmacol Ther 207:107455. https://doi.org/10.1016/j.pharmthera.2019.107455
Nakagawa Y, Yamada S (2021) A novel hypothesis on metal dyshomeostasis and mitochondrial dysfunction in amyotrophic lateral sclerosis: potential pathogenetic mechanism and therapeutic implications. Eur J Pharmacol 892:173737. https://doi.org/10.1016/j.ejphar.2020.173737
Nath S, Agholme L, Kurudenkandy FR, Granseth B, Marcusson J, Hallbeck M (2012) Spreading of neurodegenerative pathology via neuron-to-neuron transmission of β-amyloid. J Neurosci 32:8767–8777. https://doi.org/10.1523/JNEUROSCI.0615-12.2012
Nawa NE, Ando H (2019) Effective connectivity within the ventromedial prefrontal cortex-hippocampus-amygdala network during the elaboration of emotional autobiographical memories. Neuroimage 189:316–328. https://doi.org/10.1016/j.neuroimage.2019.01.042
Nedergaard M, Goldman SA (2020) Glymphatic failure as a final common pathway to dementia. Science 370:50–56. https://doi.org/10.1126/science.abb8739
Ni Y, Zhao X, Bao G, Zou L, Teng L, Wang Z, Song M, Xiong J et al (2006) Activation of β2-adrenergic receptor stimulates γ-secretase activity and accelerates amyloid plaque formation. Nat Med 12:1390–1396. https://doi.org/10.1038/nm1485
Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ (2004) Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry 161:2126–2128. https://doi.org/10.1176/appi.ajp.161.11.2126
Noh HJ, Joo EY, Kim ST, Yoon SM, Koo DL, Kim D, Lee GH, Hong SB (2012) The relationship between hippocampal volume and cognition in patients with chronic primary insomnia. J Clin Neurol 8:130–138. https://doi.org/10.3988/jcn.2012.8.2.130
Ohnishi T (1998) Iron-sulfur clusters/semiquinones in complex I. Biochim Biophys Acta 1364:186–206. https://doi.org/10.1016/s0005-2728(98)00027-9
Orsini CA, Maren S (2012) Neural and cellular mechanisms of fear and extinction memory formation. Neurosci Biobehav Rev 36:1773–1802. https://doi.org/10.1016/j.neubiorev.2011.12.014
Ott BR, Jones RN, Daiello LA, de la Monte SM, Stopa EG, Johanson CE, Denby C, Grammaset P (2018) Blood-cerebrospinal fluid barrier gradients in mild cognitive impairment and Alzheimer’s disease: relationship to inflammatory cytokines and chemokines. Front Aging Neurosci 10:245. https://doi.org/10.3389/fnagi.2018.00245
Pak K, Kim J, Kim K, Kim SJ, Kim IJ (2020) Sleep and neuroimaging. Nucl Med Mol Imaging 54:98–104. https://doi.org/10.1007/s13139-020-00636-9
Papadopoulos MC, Verkman AS (2013) Aquaporin water channels in the nervous system. Nat Rev Neurosci 14:265–277. https://doi.org/10.1038/nrn3468
Pearson HA, Peers C (2006) Physiological roles for amyloid β peptides. J Physiol 575:5–10. https://doi.org/10.1113/jphysiol.2006.111203
Peluffo H, Acarin L, Faiz M, Castellano B, Gonzalez B (2005) Cu/Zn superoxide dismutase expression in the postnatal rat brain following an excitotoxic injury. J Neuroinflammation 2:12. https://doi.org/10.1186/1742-2094-2-12
Pernègre C, Duquette A, Leclerc N (2019) Tau secretion: good and bad for neurons. Front Neurosci 13:649. https://doi.org/10.3389/fnins.2019.00649
Petrilli MA, Kranz TM, Kleinhaus K, Joe P, Getz M, Johnson P, Chao MV, Malaspina D (2017) The emerging role for zinc in depression and psychosis. Front Pharmacol 8:414. https://doi.org/10.3389/fphar.2017.00414
Pisani V, Stefani A, Pierantozzi M, Natoli S, Stanzione P, Pisani FDA (2012) Increased blood-cerebrospinal fluid transfer of albumin in advanced Parkinson’s disease. J Neuroinflammation 9:188. https://doi.org/10.1186/1742-2094-9-188
Poe GR, Foote S, Eschenko O, Johansen JP, Bouret S, Aston-Jones G, Harley CW, Manahan-Vaughan D et al (2020) Locus coeruleus: a new look at the blue spot. Nat Rev Neurosci 21:644–659. https://doi.org/10.1038/s41583-020-0360-9
Pretsch D, Rollinger JM, Schmid A, Genov M, Wöhrer T, Krenn L, Moloney M, Kasture A et al (2020) Prolongation of metallothionein induction combats Aß and α-synuclein toxicity in aged transgenic Caenorhabditis elegans. Sci Rep 10:11707. https://doi.org/10.1038/s41598-020-68561-7
Prohaska JR (2008) Role of copper transporters in copper homeostasis. Am J Clin Nutr 88:826–829. https://doi.org/10.1093/ajcn/88.3.826S
Raha AA, Biswas A, Henderson J, Chakraborty S, Holland A, Friedland RP, Mukaetova-Ladinska E, Zaman S et al (2022) Interplay of ferritin accumulation and ferroportin loss in ageing brain: implication for protein aggregation in down syndrome dementia, Alzheimer’s, and Parkinson’s diseases. Int J Mol Sci 23:1060. https://doi.org/10.3390/ijms23031060
Raha-Chowdhury R, Raha AA, Forostyak S, Zhao JW, Stott SR, Bomford A (2015) Expression and cellular localization of hepcidin mRNA and protein in normal rat brain. BMC Neurosci 16:24. https://doi.org/10.1186/s12868-015-0161-7
Rajarethinam R, DeQuardo JR, Miedler J, Arndt S, Kirbat R, Brunberg JA, Tandon R (2001) Hippocampus and amygdala in schizophrenia: assessment of the relationship of neuroanatomy to psychopathology. Psychiatry Res 108:79–87. https://doi.org/10.1016/s0925-4927(01)00120-2
Rajasekaran A, Venkatasubramanian G, Berk M, Debnath M (2015) Mitochondrial dysfunction in schizophrenia: pathways, mechanisms and implications. Neurosci Biobehav Rev 48:10–21. https://doi.org/10.1016/j.neubiorev.2014.11.005
Ramm P (1979) The locus coeruleus, catecholamines, and REM sleep: a critical review. Behav Neural Biol 25:415–448. https://doi.org/10.1016/S0163-1047(79)90212-7
Ramsey CP, Glass CA, Montgomery MB, Lindl KA, Ritson GP, Chia LA, Hamilton RL, Chu CT et al (2007) Expression of Nrf2 in neurodegenerative diseases. J Neuropathol Exp Neurol 66:75–85. https://doi.org/10.1097/nen.0b013e31802d6da9
Ranjbar E, Kasaei MS, Mohammad-Shirazi M, Nasrollahzadeh J, Rashidkhani B, Shams J, Mostafavi S-A, Mohammadi MR (2013) Effects of zinc supplementation in patients with major depression: a randomized clinical trial. Iran J Psychiatry 8:73–79
Raper D, Louveau A, Kipnis J (2016) How do meningeal lymphatic vessels drain the CNS? Trends Neurosci 39:581–586. https://doi.org/10.1016/j.tins.2016.07.001
Rasia RM, Bertoncini CW, Marsh D, Hoyer W, Cherny D, Zweckstetter M, Griesinger C, Jovin TM et al (2005) Structural characterization of copper(II) binding to alpha-synuclein: Insights into the bioinorganic chemistry of Parkinson’s disease. Proc Natl Acad Sci USA 102:4294–4299. https://doi.org/10.1073/pnas.0407881102
Roesch ZK, Tadi P (2020) Neuroanatomy, fourth ventricle. In: StatPearls. StatPearls Publishing, Treasure Island.
Ross JA, McGonigle P, Van Bockstaele EJ (2015) Locus coeruleus, norepinephrine and Aβ peptides in Alzheimer’s disease. Neurobiol Stress 2:73–84. https://doi.org/10.1016/j.ynstr.2015.09.002
Rozzini L, Lanfranchi F, Pilotto A, Catalani S, Gilberti ME, Paganelli M, Apostoli P, Padovani A (2018) Serum non-ceruloplasmin non-albumin copper elevation in mild cognitive impairment and dementia due to Alzheimer’s disease: a case control study. J Alzheimers Dis 61:907–912. https://doi.org/10.3233/JAD-170552
Saghazadeh A, Mahmoudi M, Shahrokhi S, Mojarrad M, Dastmardi M, Mirbeyk M, Rezaei N (2020) Trace elements in schizophrenia: a systematic review and meta-analysis of 39 studies (n=5151 participants). Nutr Rev 78:278–303. https://doi.org/10.1093/nutrit/nuz059
Saido T, Leissring MA (2012) Proteolytic degradation of amyloid beta-protein. Cold Spring Harb Perspect Med 2:006379–006389. https://doi.org/10.1101/cshperspect.a006379
Sakka L, Coll G, Chazal J (2011) Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis 128:309–316. https://doi.org/10.1016/j.anorl.2011.03.002
Salazar J, Mena N, Hunot S, Prigent A, Alvarez-Fischer D, Arredondo M, Duyckaerts C, Sazdovitch V et al (2008) Divalent metal transporter 1 (DMT1) contributes to neurodegeneration in animal models of Parkinson’s disease. Proc Natl Acad Sci USA 105:18578–18583. https://doi.org/10.1073/pnas.0804373105
Saman S, Kim W, Raya M, Visnick Y, Miro S, Saman S, Jackson B, McKee AC et al (2012) Exosome-associated tau is secreted in tauopathy models and is selectively phosphorylated in cerebrospinal fluid in early Alzheimer disease. J Biol Chem 287:3842–3849. https://doi.org/10.1074/jbc.M111.277061
Sandberg M, Patil J, D’Angelo B, Weber SG, Mallard C (2014) NRF2-regulation in brain health and disease: implication of cerebral inflammation. Neuropharmacology 79:298–306. https://doi.org/10.1016/j.neuropharm.2013.11.004
Sastre M, Ritchie CW, Hajji N (2015) Metal ions in Alzheimer’s disease brain. JSM Alzheimer’s Dis Related Dementia 2:1014
Sato T, Hanyu H, Hirao K, Kanetaka H, Sakurai H, Iwamoto T (2011) Efficacy of PPAR-γ agonist pioglitazone in mild Alzheimer disease. Neurobiol Aging 32:1626–1633. https://doi.org/10.1016/j.neurobiolaging.2009.10.009
Savelieff MG, Lee S, Liu Y, Lim MH (2013) Untangling amyloid-β, tau, and metals in Alzheimer’s disease. ACS Chem Biol 8:856–865. https://doi.org/10.1021/cb400080f
Selkoe DJ, Hardy J (2016) The amyloid hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med 8:595–608. https://doi.org/10.15252/emmm.201606210
Sepanjnia K, Modabbernia A, Ashrafi M, Modabbernia M-J, Akhondzadeh S (2012) Pioglitazone adjunctive therapy for moderate-to-severe major depressive disorder: randomized double-blind placebo-controlled trial. Neuropsychopharmacology 37:2093–2100. https://doi.org/10.1038/npp.2012.58
Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, Suridjan I, Kennedy JL et al (2015) Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiat 72:268–275. https://doi.org/10.1001/jamapsychiatry.2014.2427
Shafti SS, Jafarabad MS, Azizi R (2015) Amelioration of deficit syndrome of schizophrenia by norepinephrine reuptake inhibitor. Ther Adv Psychopharmacol 5:263–270. https://doi.org/10.1177/2045125315591953
Sharma SK, Chorell E, Steneberg P, Vernersson-Lindahl E, Edlund H, Wittung-Stafshede P (2015) Insulin-degrading enzyme prevents α-synuclein fibril formation in a nonproteolytical manner. Sci Rep 5:12531. https://doi.org/10.1038/srep12531
Shibata E, Sasaki M, Tohyama K, Otsuka K, Endoh J, Terayama Y, Sakai A (2008) Use of neuromelanin-sensitive MRI to distinguish schizophrenic and depressive patients and healthy individuals based on signal alterations in the substantia nigra and locus ceruleus. Biol Psychiatry 64:401–406. https://doi.org/10.1016/j.biopsych.2008.03.021
Shokri-Kojori E, Wang G, Wiers CE, Demiral SB, Guo M, Kim SW, Lindgren E, Ramirez V et al (2018) β-Amyloid accumulation in the human brain after one night of sleep deprivation. Proc Natl Acad Sci USA 115:4483–4488. https://doi.org/10.1073/pnas.1721694115
Šimić G, Leko MB, Wray S, Harrington CR, Delalle I, Jovanov-Milošević N, Bažadona D, Buée L et al (2017) Monoaminergic neuropathology in Alzheimer’s disease. Prog Neurobiol 151:101–138. https://doi.org/10.1016/j.pneurobio.2016.04.001
Sinha S, Ansell-Schultz A, Civitelli L, Hildesjö C, Larsson M, Lannfelt L, Ingelsson M, Hallbeck M (2018) Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-β oligomers. Acta Neuropathol 136:41–56. https://doi.org/10.1007/s00401-018-1868-1
Sirabella R, Valsecchi V, Anzilotti S, Cuomo O, Vinciguerra A, Cepparulo P, Brancaccio P, Guida N et al (2018) Ionic homeostasis maintenance in ALS: focus on new therapeutic targets. Front Neurosci 12:510. https://doi.org/10.3389/fnins.2018.00510
Sisó S, Jeffrey M, González L (2010) Sensory circumventricular organs in health and disease. Acta Neuropathol 120:689–705. https://doi.org/10.1007/s00401-010-0743-5
Siwek M, Dudek D, Paul IA, Sowa-Kućma M, Zieba A, Popik P, Pilc A, Nowak G (2009) Zinc supplementation augments efficacy of imipramine in treatment resistant patients: a double blind, placebo-controlled study. J Affect Disord 118:187–195. https://doi.org/10.1016/j.jad.2009.02.014
Siwek M, Dudek D, Schlegel-Zawadzka M, Morawska A, Piekoszewski W, Opoka W, Zieba A, Pilc A et al (2010) Serum zinc level in depressed patients during zinc supplementation of imipramine treatment. J Affect Disord 126:447–452. https://doi.org/10.1016/j.jad.2010.04.024
Song C-H, Kim Y-H, Jung K-I (2012) Associations of zinc and copper levels in serum and hair with sleep duration in adult women. Biol Trace Elem Res 149:16–21. https://doi.org/10.1007/s12011-012-9398-5
Squitti R, Ghidoni R, Simonelli I, Ivanova ID, Colabufo NA, Zuin M, Benussi L, Binetti G et al (2018) Copper dyshomeostasis in Wilson disease and Alzheimer’s disease as shown by serum and urine copper indicators. J Trace Elem Med Biol 45:181–188. https://doi.org/10.1016/j.jtemb.2017.11.005
Stahl SM (2013) Stahl’s essential psychopharmacology: neuroscientific basis and practical applications, 4th edn. Cambridge University Press, Cambridge
Stahl SM, Fava M, Trivedi MH, Caputo A, Shah A, Post A (2010) Agomelatine in the treatment of major depressive disorder: an 8-week, multicenter, randomized, placebo-controlled trial. J Clin Psychiatry 71:616–626. https://doi.org/10.4088/JCP.09m05471blu
Steiner JA, Angot E, Brundin P (2011) A deadly spread: cellular mechanisms of α-synuclein transfer. Cell Death Differ 18:1425–1433. https://doi.org/10.1038/cdd.2011.53
Stöckel J, Safar J, Wallace AC, Cohen FE, Prusiner SB (1998) Prion protein selectively binds copper(II) ions. Biochemistry 37:7185–7193. https://doi.org/10.1021/bi972827k
Stowell RD, Sipe GO, Dawes RP, Batchelor HN, Lordy KA, Whitelaw BS, Stoessel MB, Bidlack JM et al (2019) Noradrenergic signaling in the wakeful state inhibits microglial surveillance and synaptic plasticity in the mouse visual cortex. Nat Neurosci 22:1782–1792. https://doi.org/10.1038/s41593-019-0514-0
Styczeń K, Sowa-Kućma M, Siwek M, Dudek D, Reczyński W, Misztak P, Szewczyk B, Topór-Mądry R et al (2016) Study of the serum copper levels in patients with major depressive disorder. Biol Trace Elem Res 174:287–293. https://doi.org/10.1007/s12011-016-0720-5
Sun X-Y, Wei Y-P, Xiong Y, Wang X-C, Xie A-J, Wang X-L, Yang Y, Wang Q et al (2012) Synaptic released zinc promotes tau hyperphosphorylation by inhibition of protein phosphatase 2A (PP2A). J Biol Chem 287:11174–11182. https://doi.org/10.1074/jbc.M111.309070
Suresh Kumar PN, Andrade C, Bhakta SG, Singh NM (2007) Melatonin in schizophrenic outpatients with insomnia: a double-blind, placebo-controlled study. J Clin Psychiatry 68:237–241. https://doi.org/10.4088/jcp.v68n0208
Swardfager W, Herrmann N, Mazereeuw G, Goldberger K, Harimoto T, Lanctôt KL (2013) Zinc in depression: a meta-analysis. Biol Psychiatry 74:872–878. https://doi.org/10.1016/j.biopsych.2013.05.008
Takeda T, Iijima M, Uchihara T, Ohashi T, Seilhean D, Duyckaerts C, Uchiyama S (2015) TDP-43 pathology progression along the olfactory pathway as a possible substrate for olfactory impairment in amyotrophic lateral sclerosis. J Neuropathol Exp Neurol 74:547–556. https://doi.org/10.1097/NEN.0000000000000198
Tamietto M, de Gelder B (2010) Neural bases of the non-conscious perception of emotional signals. Nat Rev Neurosci 11:697–709. https://doi.org/10.1038/nrn2889
Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H et al (2015) Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 11:457–470. https://doi.org/10.1038/nrneurol.2015.119
Taylor DL, Jones F, Kubota ESFC, Pocock JM (2005) Stimulation of microglial metabotropic glutamate receptor mGlu2 triggers tumor necrosis factor α-induced neurotoxicity in concert with microglial-derived Fas ligand. J Neurosci 25:2952–2964. https://doi.org/10.1523/JNEUROSCI.4456-04.2005
Theodoropoulou S, Spanakos G, Baxevanis CN, Economou M, Gritzapis AD, Papamichail MP, Stefanis CN (2001) Cytokine serum levels, autologous mixed lymphocyte reaction and surface marker analysis in never medicated and chronically medicated schizophrenic patients. Schizophr Res 47:13–25. https://doi.org/10.1016/s0920-9964(00)00007-4
Theofilas P, Ehrenberg AJ, Dunlop S, Alho AT, Nguy A, Leite REP, Rodriguez RD, Mejia MB et al (2017) Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: a stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimers Dement 13:236–246. https://doi.org/10.1016/j.jalz.2016.06.2362
Tully L, Humiston J, Cash A (2020) Oxaloacetate reduces emotional symptoms in premenstrual syndrome (PMS): results of a placebo-controlled, cross-over clinical trial. Obstet Gynecol Sci 63:195–204. https://doi.org/10.5468/ogs.2020.63.2.195
Tunç S, Atagün MI, Başbuğ HS, Erel Ö (2019) Serum ceruloplasmin-ferroxidase activity in bipolar disorder is elevated compared to major depressive disorder and schizophrenia: a controlled study. Psychiatry Clin Psychopharmacol 29:307–314. https://doi.org/10.1080/24750573.2019.1584489
van Berckel BN, Bossong MG, Boellaard R, Kloet R, Schuitemaker A, Caspers E, Luurtsema G, Windhorst AD et al (2008) Microglia activation in recent-onset schizophrenia: a quantitative (R)-[11C]PK11195 positron emission tomography study. Biol Psychiatry 64:820–822. https://doi.org/10.1016/j.biopsych.2008.04.025
van der Kant R, Goldstein LSB, Ossenkoppele R (2020) Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat Rev Neurosci 21:21–35. https://doi.org/10.1038/s41583-019-0240-3
Vandekerckhove M, Cluydts R (2010) The emotional brain and sleep: an intimate relationship. Sleep Med Rev 14:219–226. https://doi.org/10.1016/j.smrv.2010.01.002
Varfolomeev EE, Ashkenazi A (2004) Tumor necrosis factor: an apoptosis JuNKie? Cell 116:491–497. https://doi.org/10.1016/s0092-8674(04)00166-7
Vassiliev V, Harris ZL, Zatta P (2005) Ceruloplasmin in neurodegenerative diseases. Brain Res Rev 49:633–640. https://doi.org/10.1016/j.brainresrev.2005.03.003
Venkataramani V, Doeppner TR, Willkommen D, Cahill CM, Xin Y, Ye G, Liu Y, Southon A et al (2018) Manganese causes neurotoxic iron accumulation via translational repression of amyloid precursor protein and H-Ferritin. J Neurochem 147:831–848. https://doi.org/10.1111/jnc.14580
Ventriglia M, Bucossi S, Panetta V, Squitti R (2012) Copper in Alzheimer’s disease: a meta-analysis of serum, plasma, and cerebrospinal fluid studies. J Alzheimers Dis 30:981–984. https://doi.org/10.3233/JAD-2012-120244
Vgontzas AN, Papanicolaou DA, Bixler EO, Lotsikas A, Zachman K, Kales A, Prolo P, Wong ML et al (1999) Circadian interleukin-6 secretion and quantity and depth of sleep. J Clin Endocrinol Metab 84:2603–2607. https://doi.org/10.1210/jcem.84.8.5894
Vilella A, Belletti D, Sauer AK, Hagmeyer S, Sarowar T, Masoni M, Stasiak N, Mulvihill JJE et al (2018) Reduced plaque size and inflammation in the APP23 mouse model for Alzheimer’s disease after chronic application of polymeric nanoparticles for CNS targeted zinc delivery. J Trace Elem Med Biol 49:210–221. https://doi.org/10.1016/j.jtemb.2017.12.006
Vilella A, Daini E, De Benedictis CA (2020) Targeting metal homeostasis as a therapeutic strategy for Alzheimer’s disease. In: Huang X (ed) Alzheimer’s disease: drug discovery. Exon Publications, Brisbane, Australia, pp 83–98. https://doi.org/10.36255/exonpublications.alzheimersdisease
Voloboueva LA, Suh SW, Swanson RA, Giffard RG (2007) Inhibition of mitochondrial function in astrocytes: implications for neuroprotection. J Neurochem 102:1383–1394. https://doi.org/10.1111/j.1471-4159.2007.04634.x
Wade AG, Farmer M, Harari G, Fund N, Laudon M, Nir T, Frydman-Marom A, Zisapel N (2014) Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: a 6-month, randomized, placebo-controlled, multicenter trial. Clin Interv Aging 9:947–961. https://doi.org/10.2147/CIA.S65625
Wallace DF (2016) The regulation of iron absorption and homeostasis. Clin Biochem Rev 37:51–62
Wan W, Jin L, Wang Z, Wang L, Fei G, Ye F, Pan X, Wang C et al (2017) Iron deposition leads to neuronal α-synuclein pathology by inducing autophagy dysfunction. Front Neurol 8:1. https://doi.org/10.3389/fneur.2017.00001
Wang Q, Li W-X, Dai S-X, Guo Y-C, Han F-F, Zheng J-J, Li G-H, Huang J-F (2017) Meta-analysis of Parkinson’s disease and Alzheimer’s disease revealed commonly impaired pathways and dysregulation of Nrf2-dependent genes. J Alzheimers Dis 56:1525–1539. https://doi.org/10.3233/JAD-161032
Wang Y, Mandelkow E (2016) Tau in physiology and pathology. Nat Rev Neurosci 17:5–21. https://doi.org/10.1038/nrn.2015.1
Wang ZX, Tan L, Wang HF, Ma J, Liu J, Tan MS, Sun JH, Zhu XC et al (2015) Serum iron, zinc, and copper levels in patients with Alzheimer’s disease: a replication study and meta-analyses. J Alzheimers Dis 47:565–581. https://doi.org/10.3233/JAD-143108
Watts DL (1988) The nutritional relationships of zinc. J Orthomol Med 3:63–67. (This article has no DOI).
Watts DL (1990) The nutritional relationships of manganese. J Orthomol Med 5:219–222. (This article has no DOI).
Weinshenker D (2018) Long road to ruin: noradrenergic dysfunction in neurodegenerative disease. Trends Neurosci 41:211–223. https://doi.org/10.1016/j.tins.2018.01.010
Weisiger RA, Fridovich I (1973) Mitochondrial superoxide dismutase: site of synthesis and intramitochondrial localization. J Biol Chem 248:4793–4796
West AK, Hidalgo J, Eddins D, Levin ED, Aschner M (2008) Metallothionein in the central nervous system: roles in protection, regeneration and cognition. Neurotoxicology 29:488–502. https://doi.org/10.1016/j.neuro.2007.12.006
Willkommen D, Lucio M, Schmitt-Kopplin P, Gazzaz M, Schroeter M, Sigaroudi A, Michalke B (2018) Species fractionation in a case-control study concerning Parkinson’s disease: Cu-amino acids discriminate CSF of PD from controls. J Trace Elem Med Biol 49:164–170. https://doi.org/10.1016/j.jtemb.2018.01.005
Winslow JWW, Limesand KH, Zhao N (2020) The Functions of ZIP8, ZIP14, and ZnT10 in the Regulation of Systemic Manganese Homeostasis. Int J Mol Sci 21:3304. https://doi.org/10.3390/ijms21093304
Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET (2000) Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry 157:16–25. https://doi.org/10.1176/ajp.157.1.16
Wong BX, Hung YH, Bush AI, Duce JA (2014) Metals and cholesterol: two sides of the same coin in Alzheimer’s disease pathology. Front Aging Neurosci 6:91. https://doi.org/10.3389/fnagi.2014.00091
Xie L, Kang H, Xu O, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ et al (2013) Sleep drives metabolite clearance from the adult brain. Science 342:373–377. https://doi.org/10.1126/science.1241224
Xu B, Wu SW, Lu CW, Deng Y, Liu W, Wei YG, Yang TY, Xu ZF (2013) Oxidative stress involvement in manganese-induced alpha-synuclein oligomerization in organotypic brain slice cultures. Toxicology 305:71–78. https://doi.org/10.1016/j.tox.2013.01.006
Xu J, Church SJ, Patassini S, Begley P, Waldvogel HJ, Curtis MA, Faull RLM, Unwin RD (2017) Evidence for widespread, severe brain copper deficiency in Alzheimer’s dementia. Metallomics 9:1106–1119. https://doi.org/10.1039/c7mt00074j
Yan X, Uronen R-L, Huttunen HJ (2020) The interaction of α-synuclein and Tau: a molecular conspiracy in neurodegeneration? Semin Cell Dev Biol 99:55–64. https://doi.org/10.1016/j.semcdb.2018.05.005
Yang L, Yin T, Liu Y, Sun J, Zhou Y, Liu J (2016) Gold nanoparticle-capped mesoporous silica-based H2O2-responsive controlled release system for Alzheimer’s disease treatment. Acta Biomater 46:177–190. https://doi.org/10.1016/j.actbio.2016.09.010
Yanik M, Kocyigit A, Tutkun H, Vural H, Herken H (2004) Plasma manganese, selenium, zinc, copper, and iron concentrations in patients with schizophrenia. Biol Trace Elem Res 98:109–117. https://doi.org/10.1385/BTER:98:2:109
Yu CA, Yu L (1993) Mitochondrial ubiquinol-cytochrome c reductase complex: crystallization and protein: ubiquinone interaction. J Bioenerg Biomembr 25:259–273. https://doi.org/10.1007/BF00762587
Zhao H-W, Lin J, Wang X-B, Cheng X, Wang J-Y, Hu B-L, Zhang Y, Zhang X et al (2013) Assessing plasma levels of selenium, copper, iron and zinc in patients of Parkinson’s disease. PLoS ONE 8:e83060. https://doi.org/10.1371/journal.pone.0083060
Zhong M, Wang X, Xiao J, Yi J, Zhu X, Liao J, Wang W, Yao S (2011) Amygdala hyperactivation and prefrontal hypoactivation in subjects with cognitive vulnerability to depression. Biol Psychol 88:233–242. https://doi.org/10.1016/j.biopsycho.2011.08.007
Zhou F, Chen S, Xiong J, Li Y, Qu L (2012) Luteolin reduces zinc-induced tau phosphorylation at Ser262/356 in an ROS-dependent manner in SH-SY5Y cells. Biol Trace Elem Res 149:273–279. https://doi.org/10.1007/s12011-012-9411-z
Zimbrean PC, Schilsky ML (2014) Psychiatric aspects of Wilson disease: a review. Gen Hosp Psychiatry 36:53–62. https://doi.org/10.1016/j.genhosppsych.2013.08.007
Acknowledgements
The authors acknowledge Professor S. J. Enna (University of Kansas Medical Center, Kansas City, KS, USA) for invaluable comments on an early draft of this report.
Funding
No external funding was expended in the preparation of this work.
Author information
Authors and Affiliations
Contributions
YN and SY had the idea for the article and performed the literature search. The first draft of the manuscript was written by YN. Both authors revised, discussed, and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Research Involving Human Participants and/or Animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nakagawa, Y., Yamada, S. The Relationships Among Metal Homeostasis, Mitochondria, and Locus Coeruleus in Psychiatric and Neurodegenerative Disorders: Potential Pathogenetic Mechanism and Therapeutic Implications. Cell Mol Neurobiol 43, 963–989 (2023). https://doi.org/10.1007/s10571-022-01234-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-022-01234-3