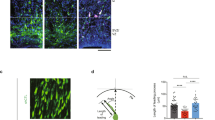
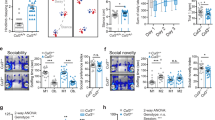
Abstract
1p34.2p34.3 deletion syndrome is characterized by an increased risk for autism. Microtubule Actin Crosslinking Factor 1 (MACF1) is one candidate gene for this syndrome. It is unclear, however, how MACF1 deletion is linked to brain development and neurodevelopmental deficits. Here we report on Macf1 deletion in the developing mouse cerebral cortex, focusing on radial glia polarity and morphological integrity, as these are critical factors in brain formation. We found that deleting Macf1 during cortical development resulted in double cortex/subcortical band heterotopia as well as disrupted cortical lamination. Macf1-deleted radial progenitors showed increased proliferation rates compared to control cells but failed to remain confined within their defined proliferation zone in the developing brain. The overproliferation of Macf1-deleted radial progenitors was associated with elevated cell cycle speed and re-entry. Microtubule stability and actin polymerization along the apical ventricular area were decreased in the Macf1 mutant cortex. Correspondingly, there was a disconnection between radial glial fibers and the apical and pial surfaces. Finally, we observed that Macf1-mutant mice exhibited social deficits and aberrant emotional behaviors. Together, these results suggest that MACF1 plays a critical role in cortical progenitor proliferation and localization by promoting glial fiber stabilization and polarization. Our findings may provide insights into the pathogenic mechanism underlying the 1p34.2p34.3 deletion syndrome.









Similar content being viewed by others
Data Availability
Data and materials will be available depending on request and availability.
References
Aktas D, Utine EG, Mrasek K, Weise A, von Eggeling F, Yalaz K, Posorski N, Akarsu N, Alikasifoglu M, Liehr T, Tuncbilek E (2010) Derivative chromosome 1 and GLUT1 deficiency syndrome in a sibling pair. Mol Cytogenet 3(1):10. https://doi.org/10.1186/1755-8166-3-10
Bailey KR, Crawley JN (2009) Anxiety-related behaviors in mice. In: Methods of Behavior Analysis in Neuroscience. 2nd edn, Chap 5. CRC Press/Taylor & Francis, Boca Raton, FL
Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB (2005) A developmental and genetic classification for malformations of cortical development. Neurology 65(12):1873?1887. https://doi.org/10.1212/01.wnl.0000183747.05269.2d
Belzung C (2001) Rodent models of anxiety-like behaviors: are they predictive for compounds acting via non-benzodiazepine mechanisms? Curr Opin Investig Drugs 2(8):1108?1111
Bodensteiner J, Schaefer GB, Breeding L, Cowan L (1994) Hypoplasia of the corpus callosum: a study of 445 consecutive MRI scans. J Child Neurol 9(1):47?49. https://doi.org/10.1177/088307389400900111
Chenn A, Walsh CA (2002) Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science 297(5580):365?369. https://doi.org/10.1126/science.1074192
Crawley JN (1999) Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res 835(1):18?26. https://doi.org/10.1016/s0006-8993(98)01258-x
Dagklis T, Papageorgiou E, Siomou E, Paspaliaris V, Zerva C, Chatzis P, Thomaidis L, Manolakos E, Papoulidis I (2016) Prenatal diagnosis of 1p34.3 interstitial microdeletion by aCGH in a fetus with jaw bone abnormalities. Mol Cytogenet 9:77. https://doi.org/10.1186/s13039-016-0288-y
Deacon RM (2013) Measuring the strength of mice. J Vis Exp. https://doi.org/10.3791/2610
Dehay C, Kennedy H (2007) Cell-cycle control and cortical development. Nat Rev Neurosci 8(6):438?450. https://doi.org/10.1038/nrn2097
Dobyns WB, Andermann E, Andermann F, Czapansky-Beilman D, Dubeau F, Dulac O, Guerrini R, Hirsch B, Ledbetter DH, Lee NS, Motte J, Pinard JM, Radtke RA, Ross ME, Tampieri D, Walsh CA, Truwit CL (1996) X-linked malformations of neuronal migration. Neurology 47(2):331?339
Dulawa SC, Holick KA, Gundersen B, Hen R (2004) Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 29(7):1321?1330. https://doi.org/10.1038/sj.npp.1300433
Geschwind DH (2011) Genetics of autism spectrum disorders. Trends Cogn Sci 15(9):409?416. https://doi.org/10.1016/j.tics.2011.07.003
Geschwind DH, Rakic P (2013) Cortical evolution: judge the brain by its cover. Neuron 80(3):633?647. https://doi.org/10.1016/j.neuron.2013.10.045
Goebbels S, Bormuth I, Bode U, Hermanson O, Schwab MH, Nave KA (2006) Genetic targeting of principal neurons in neocortex and hippocampus of NEX-Cre mice. Genesis 44(12):611?621. https://doi.org/10.1002/dvg.20256
Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR (2002) Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci 22(15):6309?6314
Gotz M, Huttner WB (2005) The cell biology of neurogenesis. Nat Rev Mol Cell Biol 6(10):777?788. https://doi.org/10.1038/nrm1739
Huttner WB, Kosodo Y (2005) Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr Opin Cell Biol 17(6):648?657. https://doi.org/10.1016/j.ceb.2005.10.005
Iwasato T, Nomura R, Ando R, Ikeda T, Tanaka M, Itohara S (2004) Dorsal telencephalon-specific expression of Cre recombinase in PAC transgenic mice. Genesis 38(3):130?138. https://doi.org/10.1002/gene.20009
Jacher JE, Innis JW (2018) Interstitial microdeletion of the 1p34.3p34.2 region. Mol Genet Genom Med. https://doi.org/10.1002/mgg3.409
Jeret JS, Serur D, Wisniewski K, Fisch C (1985) Frequency of agenesis of the corpus callosum in the developmentally disabled population as determined by computerized tomography. Pediatr Neurosci 12(2):101?103
Jung EM, Ka M, Kim WY (2016) Loss of GSK-3 causes abnormal astrogenesis and behavior in mice. Mol Neurobiol 53(6):3954?3966. https://doi.org/10.1007/s12035-015-9326-8
Jung EM, Moffat JJ, Liu J, Dravid SM, Gurumurthy CB, Kim WY (2017) Arid1b haploinsufficiency disrupts cortical interneuron development and mouse behavior. Nat Neurosci 20(12):1694?1707. https://doi.org/10.1038/s41593-017-0013-0
Ka M, Kim WY (2016) Microtubule-actin crosslinking factor 1 is required for dendritic arborization and axon outgrowth in the developing brain. Mol Neurobiol 53(9):6018?6032. https://doi.org/10.1007/s12035-015-9508-4
Ka M, Kim WY (2018) ANKRD11 associated with intellectual disability and autism regulates dendrite differentiation via the BDNF/TrkB signaling pathway. Neurobiol Dis 111:138?152. https://doi.org/10.1016/j.nbd.2017.12.008
Ka M, Condorelli G, Woodgett JR, Kim WY (2014a) mTOR regulates brain morphogenesis by mediating GSK3 signaling. Development 141(21):4076?4086. https://doi.org/10.1242/dev.108282
Ka M, Jung EM, Mueller U, Kim WY (2014b) MACF1 regulates the migration of pyramidal neurons via microtubule dynamics and GSK-3 signaling. Dev Biol 395(1):4?18. https://doi.org/10.1016/j.ydbio.2014.09.009
Ka M, Chopra DA, Dravid SM, Kim WY (2016a) Essential roles for ARID1B in dendritic arborization and spine morphology of developing pyramidal neurons. J Neurosci 36(9):2723?2742. https://doi.org/10.1523/JNEUROSCI.2321-15.2016
Ka M, Kook YH, Liao K, Buch S, Kim WY (2016b) Transactivation of TrkB by Sigma-1 receptor mediates cocaine-induced changes in dendritic spine density and morphology in hippocampal and cortical neurons. Cell Death Dis 7(10):e2414. https://doi.org/10.1038/cddis.2016.319
Ka M, Moffat JJ, Kim WY (2017a) MACF1 controls migration and positioning of cortical GABAergic interneurons in mice. Cereb Cortex 27(12):5525?5538. https://doi.org/10.1093/cercor/bhw319
Ka M, Smith AL, Kim WY (2017b) MTOR controls genesis and autophagy of GABAergic interneurons during brain development. Autophagy 13(8):1348?1363. https://doi.org/10.1080/15548627.2017.1327927
Kappeler C, Dhenain M, Phan Dinh Tuy F, Saillour Y, Marty S, Fallet-Bianco C, Souville I, Souil E, Pinard JM, Meyer G, Encha-Razavi F, Volk A, Beldjord C, Chelly J, Francis F (2007) Magnetic resonance imaging and histological studies of corpus callosal and hippocampal abnormalities linked to doublecortin deficiency. J Comp Neurol 500(2):239?254. https://doi.org/10.1002/cne.21170
Kee N, Sivalingam S, Boonstra R, Wojtowicz JM (2002) The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J Neurosci Methods 115(1):97?105. https://doi.org/10.1016/s0165-0270(02)00007-9
Kim WY, Horbinski C, Sigurdson W, Higgins D (2004) Proteasome inhibitors suppress formation of polyglutamine-induced nuclear inclusions in cultured postmitotic neurons. J Neurochem 91(5):1044?1056. https://doi.org/10.1111/j.1471-4159.2004.02788.x
Kim WY, Zhou FQ, Zhou J, Yokota Y, Wang YM, Yoshimura T, Kaibuchi K, Woodgett JR, Anton ES, Snider WD (2006) Essential roles for GSK-3s and GSK-3-primed substrates in neurotrophin-induced and hippocampal axon growth. Neuron 52(6):981?996. https://doi.org/10.1016/j.neuron.2006.10.031
Kim WY, Wang X, Wu Y, Doble BW, Patel S, Woodgett JR, Snider WD (2009) GSK-3 is a master regulator of neural progenitor homeostasis. Nat Neurosci 12(11):1390?1397. https://doi.org/10.1038/nn.2408
Kumar RA, Sudi J, Babatz TD, Brune CW, Oswald D, Yen M, Nowak NJ, Cook EH, Christian SL, Dobyns WB (2010) A de novo 1p34.2 microdeletion identifies the synaptic vesicle gene RIMS3 as a novel candidate for autism. J Med Genet 47(2):81?90. https://doi.org/10.1136/jmg.2008.065821
Lee JA, Lupski JR (2006) Genomic rearrangements and gene copy-number alterations as a cause of nervous system disorders. Neuron 52(1):103?121. https://doi.org/10.1016/j.neuron.2006.09.027
Lupski JR, Stankiewicz P (2005) Genomic disorders: molecular mechanisms for rearrangements and conveyed phenotypes. PLoS Genet 1(6):e49. https://doi.org/10.1371/journal.pgen.0010049
May-Simera HL, Gumerson JD, Gao C, Campos M, Cologna SM, Beyer T, Boldt K, Kaya KD, Patel N, Kretschmer F, Kelley MW, Petralia RS, Davey MG, Li T (2016) Loss of MACF1 abolishes ciliogenesis and disrupts apicobasal polarity establishment in the retina. Cell Rep 17(5):1399?1413. https://doi.org/10.1016/j.celrep.2016.09.089
Mefford HC, Batshaw ML, Hoffman EP (2012) Genomics, intellectual disability, and autism. N Engl J Med 366(8):733?743. https://doi.org/10.1056/NEJMra1114194
Messier PE, Auclair C (1973) Inhibition of nuclear migration in the absence of microtubules in the chick embryo. J Embryol Exp Morphol 30(3):661?671
Moffat JJ, Ka M, Jung EM, Smith AL, Kim WY (2017) The role of MACF1 in nervous system development and maintenance. Semin Cell Dev Biol. https://doi.org/10.1016/j.semcdb.2017.05.020
Noordstra I, Liu Q, Nijenhuis W, Hua S, Jiang K, Baars M, Remmelzwaal S, Martin M, Kapitein LC, Akhmanova A (2016) Control of apico-basal epithelial polarity by the microtubule minus-end-binding protein CAMSAP3 and spectraplakin ACF7. J Cell Sci 129(22):4278?4288. https://doi.org/10.1242/jcs.194878
Pang T, Atefy R, Sheen V (2008) Malformations of cortical development. Neurologist 14(3):181?191. https://doi.org/10.1097/NRL.0b013e31816606b9
Raybaud C (2010) The corpus callosum, the other great forebrain commissures, and the septum pellucidum: anatomy, development, and malformation. Neuroradiology 52(6):447?477. https://doi.org/10.1007/s00234-010-0696-3
Smith AL, Jung EM, Jeon BT, Kim WY (2020) Arid1b haploinsufficiency in parvalbumin- or somatostatin-expressing interneurons leads to distinct ASD-like and ID-like behavior. Sci Rep 10(1):7834. https://doi.org/10.1038/s41598-020-64066-5
Takenouchi T, Hashida N, Torii C, Kosaki R, Takahashi T, Kosaki K (2014) 1p34.3 deletion involving GRIK3: further clinical implication of GRIK family glutamate receptors in the pathogenesis of developmental delay. Am J Med Genet A 164A(2):456?460. https://doi.org/10.1002/ajmg.a.36240
Tokita MJ, Chow PM, Mirzaa G, Dikow N, Maas B, Isidor B, Le Caignec C, Penney LS, Mazzotta G, Bernardini L, Filippi T, Battaglia A, Donti E, Earl D, Prontera P (2015) Five children with deletions of 1p34.3 encompassing AGO1 and AGO3. Eur J Hum Genet 23(6):761?765. https://doi.org/10.1038/ejhg.2014.202
Tomasch J (1954) Size, distribution, and number of fibres in the human corpus callosum. Anat Rec 119(1):119?135
Toyo-oka K, Wachi T, Hunt RF, Baraban SC, Taya S, Ramshaw H, Kaibuchi K, Schwarz QP, Lopez AF, Wynshaw-Boris A (2014) 14?3-3epsilon and zeta regulate neurogenesis and differentiation of neuronal progenitor cells in the developing brain. J Neurosci 34(36):12168?12181. https://doi.org/10.1523/JNEUROSCI.2513-13.2014
Vermeer S, Koolen DA, Visser G, Brackel HJ, van der Burgt I, de Leeuw N, Willemsen MA, Sistermans EA, Pfundt R, de Vries BB (2007) A novel microdeletion in 1(p34.2p34.3), involving the SLC2A1 (GLUT1) gene, and severe delayed development. Dev Med Child Neurol 49(5):380?384. https://doi.org/10.1111/j.1469-8749.2007.00380.x
Wu X, Shen QT, Oristian DS, Lu CP, Zheng Q, Wang HW, Fuchs E (2011) Skin stem cells orchestrate directional migration by regulating microtubule-ACF7 connections through GSK3beta. Cell 144(3):341?352. https://doi.org/10.1016/j.cell.2010.12.033
Funding
Research reported in this publication was supported by an award from the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number R01NS091220 to WYK and a grant from the National Research Foundation of Korea under Grant Number NRF-2019R1A2C1009006 to MK.
Author information
Authors and Affiliations
Contributions
MK, JJM, and WK conceived, designed, performed, and analyzed the experiments. MK performed and analyzed the majority of the imaging experiments. JJM performed and analyzed the majority of the behavior experiments. MK, JM, and WK wrote the paper. WK supervised the work.
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no competing financial interests.
Ethical Approval
Mice were handled in accordance with the animal protocol approved by the Institutional Animal Care and Use Committees (IACUC) at the University of Nebraska Medical Center and Kent State University. All experimental procedures met National Institutes of Health guidelines for the care and use of laboratory animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10571_2021_1088_MOESM1_ESM.pdf
Supplementary Fig. 1. Relative brain size in Macf1-cKO mice. Relative brain weights and sizes were examined in control and Macf1-cKO mice. Their brain weights and sizes were normalized by whole body weights. N= 5 mice for each condition. Statistical significance was determined by two-tailed Student?s t-test. Data are shown as relative changes versus controls. *p < 0.05, **p < 0.01. Supplementary Fig. 2. Abnormal callosal axon development in the Macf1-cKO corpus callosum. Nissl staining of rostral and caudal areas in the coronal brain sections from 2-month-old control and Macf1-cKO mice. Stars indicate the corpus callosum. Scale bars: 200?m. The third panels show the dotted rectangular areas in the middle panels. (PDF 506 kb)
Rights and permissions
About this article
Cite this article
Ka, M., Moffat, J.J. & Kim, WY. MACF1, Involved in the 1p34.2p34.3 Microdeletion Syndrome, is Essential in Cortical Progenitor Polarity and Brain Integrity. Cell Mol Neurobiol 42, 2187–2204 (2022). https://doi.org/10.1007/s10571-021-01088-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-021-01088-1