Abstract
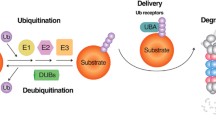
Tau is a microtubule-associated protein with an intrinsically unstructured conformation. Tau is subjected to several pathological post-translational modifications (PTMs), leading to its loss of interaction with microtubules and accumulation as neurofibrillary tangles (NFTs) in neurons. Tau aggregates impede functions of endoplasmic reticulum and mitochondria leading to the generation of oxidative stress and in turn amplifying the Tau aggregation. Tau is channelled to chaperones for folding into their native form, which otherwise causes its degradation and clearance. Cellular response triggers the activation of ubiquitin–proteasome system or autophagy to facilitate Tau degradation, based on the PTMs or mutations associated with Tau. Further, autophagy can be selective where Hsc70 interacts with Tau in monomeric, oligomeric and aggregated form and drives its clearance by chaperone-mediated autophagy pathway (CMA). Lysosome-associated membrane proteins-2A (LAMP-2A) is the key player of CMA that recognises Hsc70-Tau complex and triggers the downstream cascade. Thus, it becomes challenging for mutant Tau to be cleared by CMA as it loses its affinity for Hsc70 and LAMP-2A. In such a scenario, Tau might be degraded by macroautophagy otherwise sequestered by aggresomes. Henceforth, the degradation of Tau and its blockage that is associated with various PTMs of Tau would explain the dynamics of Tau degradation or accumulation in AD. Further, unveiling the role of accessory proteins involved in these degradation pathways would help in understanding their loss of function and preventing Tau clearance.


Similar content being viewed by others
Abbreviations
- AD:
-
Alzheimer’s disease
- Atg:
-
Autophagy-related proteins
- BAG:
-
BCL2-associated athanogene
- Bip:
-
Binding immunoglobulin protein
- CHIP:
-
Carboxyl terminus of the Hsc70-interacting protein
- CMA:
-
Chaperone-mediated autophagy
- Drp1:
-
Dynamin-like protein 1
- ER:
-
Endoplasmic reticulum
- ERAD:
-
Endoplasmic reticulum-associated degradation
- GSK-3β:
-
Glycogen synthase kinase-3 β
- IDP:
-
Intrinsically disordered protein
- HSF1:
-
Heat shock factor 1
- LAMP-2A:
-
Lysosome-associated membrane proteins-2A
- LC3B:
-
Microtubule-associated proteins 1A/1B light chain 3B
- NFTs:
-
Neurofibrillary tangles
- PTMs:
-
Post-translational modifications
- ROS:
-
Reactive oxygen species
- UPS:
-
Ubiquitin–proteasome system
References
Abisambra JF, Jinwal UK, Blair LJ, O'Leary JC, Li Q, Brady S, Wang L, Guidi CE, Zhang B, Nordhues BA (2013) Tau accumulation activates the unfolded protein response by impairing endoplasmic reticulum-associated degradation. J Neurosci 33(22):9498–9507
Agarraberes FA, Dice JF (2001) A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J Cell Sci 114(13):2491–2499
Andreadis A (2005) Tau gene alternative splicing: expression patterns, regulation and modulation of function in normal brain and neurodegenerative diseases. Biochim Et Biophys Acta (BBA) Mol Basis Dis 173(2):91–103
Avila J, Lucas JJ, Perez M, Hernandez F (2004) Role of tau protein in both physiological and pathological conditions. Physiol Rev 84(2):361–384
Bandyopadhyay U, Kaushik S, Varticovski L, Cuervo AM (2008) The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol Cell Biol 28(18):5747–5763
Baughman HE, Clouser AF, Klevit RE, Nath A (2018) HspB1 and Hsc70 chaperones engage distinct tau species and have different inhibitory effects on amyloid formation. J Biol Chem 293(8):2687–2700
Bento CF, Renna M, Ghislat G, Puri C, Ashkenazi A, Vicinanza M, Menzies FM, Rubinsztein DC (2016) Mammalian autophagy: how does it work? Annu Rev Biochem 85:685–713
Caballero B, Wang Y, Diaz A, Tasset I, Juste YR, Stiller B, Mandelkow EM, Mandelkow E, Cuervo AM (2018) Interplay of pathogenic forms of human tau with different autophagic pathways. Aging Cell 17(1):e12692
Cheng Y, Bai F (2018) The association of tau with mitochondrial dysfunction in alzheimer's disease. Front Neurosci 12:163
Chiang H-L, Plant C, Dice J (1989) A role for a 70-kilodalton heat shock protein in lysosomal degradation of intracellular proteins. Science 246(4928):382–385
Ciechanover A, Kwon YT (2015) Degradation of misfolded proteins in neurodegenerative diseases: therapeutic targets and strategies. Exp Mol Med 47(3):e147–e147
Cohen TJ, Guo JL, Hurtado DE, Kwong LK, Mills IP, Trojanowski JQ, Lee VM (2011) The acetylation of tau inhibits its function and promotes pathological tau aggregation. Nat Commun 2(1):1–9
Course MM, Wang X (2016) Transporting mitochondria in neurons. F1000Research. https://doi.org/10.12688/f1000research.7864.1
Cuervo AM, Wong E (2014) Chaperone-mediated autophagy: roles in disease and aging. Cell Res 24(1):92–104
Cuervo AM, Knecht E, Terlecky SR, Dice JF (1995) Activation of a selective pathway of lysosomal proteolysis in rat liver by prolonged starvation. Am J Physiol Cell Physiol 269(5):C1200–C1208
Dice JF (1990) Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem Sci 15(8):305–309
Eskelinen E-L, Schmidt CK, Neu S, Willenborg M, Fuertes G, Salvador N, Tanaka Y, Lullmann-Rauch R, Hartmann D, Heeren J (2004) Disturbed cholesterol traffic but normal proteolytic function in LAMP-1/LAMP-2 double-deficient fibroblasts. Mol Biol Cell 15(7):3132–3145
Fernández-Fernández MR, Gragera M, Ochoa-Ibarrola L, Quintana-Gallardo L, Valpuesta JM (2017) Hsp70–a master regulator in protein degradation. FEBS Lett 591(17):2648–2660
Ferrari L, Geerts WJ, van Wezel M, Kos R, Konstantoulea A, van Bezouwen LS, Forster FG, Rudiger SG (2018) Human chaperones untangle fibrils of the Alzheimer protein Tau. Biorxiv. https://doi.org/10.1101/426650
Flach K, Hilbrich I, Schiffmann A, Gärtner U, Krüger M, Leonhardt M, Waschipky H, Wick L, Arendt T, Holzer M (2012) Tau oligomers impair artificial membrane integrity and cellular viability. J Biol Chem 287(52):43223–43233
Gamerdinger M, Hajieva P, Kaya AM, Wolfrum U, Hartl FU, Behl C (2009) Protein quality control during aging involves recruitment of the macroautophagy pathway by BAG3. EMBO J 28(7):889–901
Gao Y, Tan L, Yu J-T, Tan L (2018) Tau in Alzheimer's disease: mechanisms and therapeutic strategies. Curr Alzheimer Res 15(3):283–300
Gong Z, Tasset I, Diaz A, Anguiano J, Tas E, Cui L, Kuliawat R, Liu H, Kühn B, Cuervo AM (2018) Humanin is an endogenous activator of chaperone-mediated autophagy. J Cell Biol 217(2):635–647
Harris H, Rubinsztein DC (2012) Control of autophagy as a therapy for neurodegenerative disease. Nat Rev Neurol 8(2):108
Ho Y-S, Yang X, Lau JC-F, Hung CH-L, Wuwongse S, Zhang Q, Wang J, Baum L, So K-F, Chang RC-C (2012) Endoplasmic reticulum stress induces tau pathology and forms a vicious cycle: implication in Alzheimer's disease pathogenesis. J Alzheimer's Dis 28(4):839–854
Huynh KK, Eskelinen EL, Scott CC, Malevanets A, Saftig P, Grinstein S (2007) LAMP proteins are required for fusion of lysosomes with phagosomes. EMBO J 26(2):313–324
Ingram EM, Spillantini MG (2002) Tau gene mutations: dissecting the pathogenesis of FTDP-17. Trends Mol Med 8(12):555–562
Inoue K, Rispoli J, Kaphzan H, Klann E, Chen EI, Kim J, Komatsu M, Abeliovich A (2012) Macroautophagy deficiency mediates age-dependent neurodegeneration through a phospho-tau pathway. Mol Neurodegener 7(1):48
Ji C, Tang M, Zeidler C, Höhfeld J, Johnson GV (2019) BAG3 and SYNPO (synaptopodin) facilitate phospho-MAPT/Tau degradation via autophagy in neuronal processes. Autophagy 15(7):1199–1213
Jing K, Lim K (2012) Why is autophagy important in human diseases? Exp Mol Med 44(2):69–72
Johansen T, Lamark T (2011) Selective autophagy mediated by autophagic adapter proteins. Autophagy 7(3):279–296
Kaushik S, Massey AC, Mizushima N, Cuervo AM (2008) Constitutive activation of chaperone-mediated autophagy in cells with impaired macroautophagy. Mol Biol Cell 19(5):2179–2192
Keck S, Nitsch R, Grune T, Ullrich O (2003) Proteasome inhibition by paired helical filament-tau in brains of patients with Alzheimer's disease. J Neurochem 85(1):115–122
Khurana V, Elson-Schwab I, Fulga TA, Sharp KA, Loewen CA, Mulkearns E, Tyynelä J, Scherzer CR, Feany MB (2010) Lysosomal dysfunction promotes cleavage and neurotoxicity of tau in vivo. PLoS Genet 6(7):e1001026
Koga H, Cuervo AM (2011) Chaperone-mediated autophagy dysfunction in the pathogenesis of neurodegeneration. Neurobiol Dis 43(1):29–37
Lacovich V, Espindola SL, Alloatti M, Devoto VP, Cromberg LE, Čarná ME, Forte G, Gallo J-M, Bruno L, Stokin GB (2017) Tau isoforms imbalance impairs the axonal transport of the amyloid precursor protein in human neurons. J Neurosci 37(1):58–69
Li W, Yang Q, Mao Z (2011) Chaperone-mediated autophagy: machinery, regulation and biological consequences. Cell Mol Life Sci 68(5):749–763
Liu Z-C, Fu Z-Q, Song J, Zhang J-Y, Wei Y-P, Chu J, Han L, Qu N, Wang J-Z, Tian Q (2012) Bip enhanced the association of GSK-3β with tau during ER stress both in vivo and in vitro. J Alzheimer's Dis 29(4):727–740
Loeffler DA, Klaver AC, Coffey MP, Aasly JO (2018) Cerebrospinal fluid concentration of key autophagy protein Lamp2 changes little during Normal aging. Front Aging Neurosci 10:130
Lovell MA, Xiong S, Xie C, Davies P, Markesbery WR (2004) Induction of hyperphosphorylated tau in primary rat cortical neuron cultures mediated by oxidative stress and glycogen synthase kinase-3. J Alzheimer's Dis 6(6):659–671
Luo H-B, Xia Y-Y, Shu X-J, Liu Z-C, Feng Y, Liu X-H, Yu G, Yin G, Xiong Y-S, Zeng K (2014) SUMOylation at K340 inhibits tau degradation through deregulating its phosphorylation and ubiquitination. Proc Natl Acad Sci 111(46):16586–16591
Manczak M, Reddy PH (2012) Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer's disease neurons: implications for mitochondrial dysfunction and neuronal damage. Hum Mol Genet 21(11):2538–2547
Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM (2006) Consequences of the selective blockage of chaperone-mediated autophagy. Proc Natl Acad Sci 103(15):5805–5810
Massey AC, Follenzi A, Kiffin R, Zhang C, Cuervo AM (2008) Early cellular changes after blockage of chaperone-mediated autophagy. Autophagy 4(4):442–456
Paonessa F, Evans LD, Solanki R, Larrieu D, Wray S, Hardy J, Jackson SP, Livesey FJ (2019) Microtubules deform the nuclear membrane and disrupt nucleocytoplasmic transport in tau-mediated frontotemporal dementia. Cell Rep 26(3):582–593
Pérez MJ, Jara C, Quintanilla RA (2018) Contribution of tau pathology to mitochondrial impairment in neurodegeneration. Front Neurosci 12:441
Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, Grover A, De Lucia M, McGowan E, Lewis J, Prihar G (2004) CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum Mol Genet 13(7):703–714
Shahpasand K, Uemura I, Saito T, Asano T, Hata K, Shibata K, Toyoshima Y, Hasegawa M, Hisanaga S-i (2012) Regulation of mitochondrial transport and inter-microtubule spacing by tau phosphorylation at the sites hyperphosphorylated in Alzheimer's disease. J Neurosci 32(7):2430–2441
Stürner E, Behl C (2017) The role of the multifunctional BAG3 protein in cellular protein quality control and in disease. Front Mol Neurosci 10:177
Taylor IR, Ahmad A, Wu T, Nordhues BA, Bhullar A, Gestwicki JE, Zuiderweg ER (2018) The disorderly conduct of Hsc70 and its interaction with the Alzheimer's-related Tau protein. J Biol Chem 293(27):10796–10809
Terlecky SR, Chiang H, Olson TS, Dice JF (1992) Protein and peptide binding and stimulation of in vitro lysosomal proteolysis by the 73-kDa heat shock cognate protein. J Biol Chem 267(13):9202–9209
Wang G, Mao Z (2014) Chaperone-mediated autophagy: roles in neurodegeneration. Transl Neurodegeners 3(1):20
Wang Y, Mandelkow E (2016) Tau in physiology and pathology. Nat Rev Neurosci 17(1):22
Wang D-W, Peng Z-J, Ren G-F, Wang G-X (2015) The different roles of selective autophagic protein degradation in mammalian cells. Oncotarget 6(35):37098
Wang Y, Martinez-Vicente M, Krüger U, Kaushik S, Wong E, Mandelkow E-M, Cuervo AM, Mandelkow E (2009) Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Hum Mol Genet 18(21):4153–4170
Wu H, Chen S, Ammar A-B, Xu J, Wu Q, Pan K, Zhang J, Hong Y (2015) Crosstalk between macroautophagy and chaperone-mediated autophagy: implications for the treatment of neurological diseases. Mol Neurobiol 52(3):1284–1296
Yang Z, Klionsky DJ (2010) Eaten alive: a history of macroautophagy. Nat Cell Biol 12(9):814–822
Funding
This project is supported in part by grant from in-house CSIR-National Chemical Laboratory Grant MLP029526.
Author information
Authors and Affiliations
Contributions
NV and SC prepared the initial draft of the paper. SC conceived the idea of the work, supervised, provided resources and wrote the paper. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Research Involving Human Participants and /or Animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gorantla, N.V., Chinnathambi, S. Autophagic Pathways to Clear the Tau Aggregates in Alzheimer’s Disease. Cell Mol Neurobiol 41, 1175–1181 (2021). https://doi.org/10.1007/s10571-020-00897-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-020-00897-0