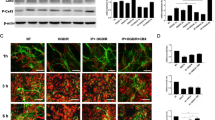
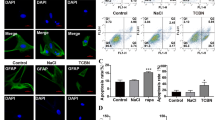
Abstract
Excitatory toxicity is still a hot topic in the study of ischemic stroke, and related research has focused mainly on neurons. Adenosine is an important neuromodulator that is known as a “biosignature” in the central nervous system (CNS). The protective effect of exogenous adenosine on neurons has been confirmed, but its mechanism remains elusive. In this study, astrocytes were pretreated with adenosine, and the effects of an A2a receptor (A2aR) inhibitor (SCH58261) and A2b receptor (A2bR) inhibitor (PSB1115) on excitatory glutamate were investigated. An oxygen glucose deprivation/reoxygenation (OGD/R) and glutamate model was generated in vitro. Post-model assessment included expression levels of glutamate transporters (glt-1), gap junction protein (Cx43) and glutamate receptor (AMPAR), Na+-K+-ATPase activity, and diffusion distance of dyes. Glutamate and glutamine contents were determined at different time points. The results showed that (1) adenosine could improve the function of Na+-K+-ATPase, upregulate the expression of glt-1, and enhance the synthesis of glutamine in astrocytes. This effect was associated with A2aR activation but not with A2bR activation. (2) Adenosine could inhibit the expression of gap junction protein (Cx43) and reduce glutamate diffusion. Inhibition of A2aR attenuated adenosine inhibition of gap junction intercellular communication (GJIC) in the OGD/R model, while it enhanced adenosine inhibition of GJIC in the glutamate model, depending on the glutamate concentration. (3) Adenosine could cause AMPAR gradually entered the nucleus from the cytoplasm, thereby reducing the expression of AMPAR on the cell membrane. Taken together, the results indicate that adenosine plays a role of anti-excitatory toxicity effect in protection against neuronal death and the functional recovery of ischemic stroke mainly by targeting astrocytes, which are closely related to A2aR. The present study provided a scientific basis for adenosine prevention and ischemic stroke treatment, thereby providing a new approach for alleviating ischemic stroke.








Similar content being viewed by others
References
Al Rahim M, Hossain MA (2013) Genetic deletion of NP1 prevents hypoxic-ischemic neuronal death via reducing AMPA receptor synaptic localization in hippocampal neurons. J Am Heart Assoc 2(1):e006098. https://doi.org/10.1161/JAHA.112.006098
Anderson MA, Ao Y, Sofroniew MV (2014) Heterogeneity of reactive astrocytes. Neurosci Lett 565:23–29
Arrubla J, Farrher E, Strippelmann J, Tse DHY, Grinberg F, Shah NJ et al (2017) Microstructural and functional correlates of glutamate concentration in the posterior cingulate cortex. J Neurosci Res 95(9):1796–1808
Atef RM, Agha AM, Abdel-Rhaman AA, Nassar NN (2018) The Ying and Yang of adenosine A1 and A2A receptors on ERK1/2 activation in a rat model of global cerebral ischemia reperfusion injury. Mol Neurobiol 55(2):1284–1298
Awasthi A, Ramachandran B, Ahmed S, Benito E, Shinoda Y, Nitzan N et al (2019) Synaptotagmin-3 drives AMPA receptor endocytosis, depression of synapse strength, and forgetting. Science 363(6422):eaav1483
Bai W, Li P, Ning YL, Peng Y, Xiong RP, Yang N et al (2018) Adenosine A2A receptor inhibition restores the normal transport of endothelial glutamate transporters in the brain. Biochem Biophys Res Commun 498(4):795–802
Bao L, Li RH, Li M, Jin MF, Li G, Han X et al (2017) Autophagy-regulated AMPAR subunit upregulation in in vitro oxygen glucose deprivation/reoxygenation-induced hippocampal injury. Brain Res 1668:65–71
Belousov AB, Fontes JD (2014) Neuronal gap junction coupling as the primary determinant of the extent of glutamate-mediated excitotoxicity. J Neural Transm (Vienna) 121(8):837–846. https://doi.org/10.1007/s00702-013-1109-7
Bissen D, Foss F, Acker-Palmer A (2019) AMPA receptors and their minions: auxiliary proteins in AMPA receptor trafficking. Cell Mol Life Sci (CMLS) 76(11):2133–2169
Bobermin LD, Arus BA, Leite MC, Souza DO, Goncalves CA, Quincozes-Santos A (2016) Gap junction intercellular communication mediates ammonia-induced neurotoxicity. Neurotox Res 29(2):314–324
Brosnan JT, Brosnan ME (2013) Glutamate: a truly functional amino acid. Amino Acids 45(3):413–418
Castillo CA, Leon DA, Ballesteros-Yanez I, Albasanz JL, Martin M (2010) Glutamate differently modulates excitatory and inhibitory adenosine receptors in neuronal and glial cells. Neurochem Int 57(1):33–42
Ceprian M, Fulton D (2019) Glial cell AMPA receptors in nervous system health, injury and disease. Int J Mol Sci 20(10):E2450. https://doi.org/10.3390/ijms20102450
Chang Y, Du C, Han L, Lv S, Zhang J, Bian G et al (2019) Enhanced AMPA receptor-mediated excitatory transmission in the rodent rostromedial tegmental nucleus following lesion of the nigrostriatal pathway. Neurochem Int 122:85–93
Chen G, Chen J, Ji X, Xi G, Zhang J (2015) Editorial for the third Pangu stroke conference. Exp Neurol 272:1–3
Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A et al (2016a) Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 533(7604):493–498
Chen YL, Zhang YN, Wang ZZ, Xu WG, Li RP, Zhang JD (2016b) Effects of adenosine metabolism in astrocytes on central nervous system oxygen toxicity. Brain Res 1635:180–189
Chen Q, Boire A, Jin X, Valiente M, Emrah EE, Lopez-Soto A et al (2017) Corrigendum: carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature 544(7648):124
Chen W, Guo Y, Yang W, Chen L, Ren D, Wu C et al (2018) Phosphorylation of connexin 43 induced by traumatic brain injury promotes exosome release. J Neurophysiol 119(1):305–311
Chung WS, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C et al (2013) Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 504(7480):394–400
Covenas R, Mangas A, Sanchez ML, Cadena D, Husson M, Geffard M (2017) Generation of specific antisera directed against D-amino acids: focus on the neuroanatomical distribution of D-glutamate and other D-amino acids. Fol Histochem Cytobiol 55(4):177–189
Dai SS, Zhou YG, Li W, An JH, Li P, Yang N et al (2010) Local glutamate level dictates adenosine A2A receptor regulation of neuroinflammation and traumatic brain injury. J Neurosci 30(16):5802–5810
Dal-Cim T, Poluceno GG, Lanznaster D, de Oliveira KA, Nedel CB, Tasca CI et al (2019) Guanosine prevents oxidative damage and glutamate uptake impairment induced by oxygen/glucose deprivation in cortical astrocyte cultures: involvement of A1 and A2A adenosine receptors and PI3K, MEK, and PKC pathways. Purinergic Signal 5(4):465–476. https://doi.org/10.1007/s11302-019-09679-w
Divito CB, Underhill SM (2014) Excitatory amino acid transporters: roles in glutamatergic neurotransmission. Neurochem Int 73:172–180
Eelen G, Dubois C, Cantelmo AR, Goveia J, Bruning U, DeRan M et al (2018) Role of glutamine synthetase in angiogenesis beyond glutamine synthesis. Nature 561(7721):63–69
Epifantseva I, Shaw RM (2018) Intracellular trafficking pathways of Cx43 gap junction channels. Biochim Biophys Biomembr 1860(1):40–47
Farhy-Tselnicker I, Allen NJ (2018) Astrocytes, neurons, synapses: a tripartite view on cortical circuit development. Neural Dev 13(1):7
Fattorini G, Ripoli C, Cocco S, Spinelli M, Mattera A, Grassi C et al (2019) Glutamate/GABA co-release selectively influences postsynaptic glutamate receptors in mouse cortical neurons. Neuropharmacology 161:107737
Frankish H (2019) News from the international stroke conference, 2019. Lancet Neurol 18(4):337
Freitas-Andrade M, Naus CC (2016) Astrocytes in neuroprotection and neurodegeneration: the role of connexin43 and pannexin1. Neuroscience 323:207–221
Freitas-Andrade M, She J, Bechberger J, Naus CC, Sin WC (2018) Acute connexin43 temporal and spatial expression in response to ischemic stroke. J Cell Commun Signal 12(1):193–204
Fusco I, Cherchi F, Catarzi D, Colotta V, Varano F, Pedata F et al (2019) Functional characterization of a novel adenosine A2B receptor agonist on short-term plasticity and synaptic inhibition during oxygen and glucose deprivation in the rat CA1 hippocampus. Brain Res Bull 151:174–180. https://doi.org/10.1016/j.brainresbull.2019.05.018
Genda EN, Jackson JG, Sheldon AL, Locke SF, Greco TM, O’Donnell JC et al (2011) Co-compartmentalization of the astroglial glutamate transporter, GLT-1, with glycolytic enzymes and mitochondria. J Neurosci 31(50):18275–18288
Goncalves FQ, Pires J, Pliassova A, Beleza R, Lemos C, Marques JM et al (2015) Adenosine A2b receptors control A1 receptor-mediated inhibition of synaptic transmission in the mouse hippocampus. Eur J Neurosci 41(7):878–888
Greer K, Chen J, Brickler T, Gourdie R, Theus MH (2017) Modulation of gap junction-associated Cx43 in neural stem/progenitor cells following traumatic brain injury. Brain Res Bull 134:38–46
Grenz A, Homann D, Eltzschig HK (2011) Extracellular adenosine: a safety signal that dampens hypoxia-induced inflammation during ischemia. Antioxid Redox Signal 15(8):2221–2234
Guillamon-Vivancos T, Gomez-Pinedo U, Matias-Guiu J (2015) Astrocytes in neurodegenerative diseases (I): function and molecular description. Neurología 30(2):119–129
Han BR, Lin SC, Espinosa K, Thorne PR, Vlajkovic SM (2019) Inhibition of the adenosine A2A receptor mitigates excitotoxic injury in organotypic tissue cultures of the rat cochlea. Cells 8(8):E877. https://doi.org/10.3390/cells8080877
Hankey GJ (2014) Secondary stroke prevention. Lancet Neurol 13(2):178–194
Hansen JC, Bjorn-Yoshimoto WE, Bisballe N, Nielsen B, Jensen AA, Bunch L (2016) Beta-sulfonamido functionalized aspartate analogues as excitatory amino acid transporter inhibitors: distinct subtype selectivity profiles arising from subtle structural differences. J Med Chem 59(19):8771–8786
Haucke V (2000) Dissecting the ins and outs of excitement: glutamate receptors on the move. Nat Neurosci 3(12):1230–1232
Hayakawa K, Esposito E, Wang X, Terasaki Y, Liu Y, Xing C et al (2016) Transfer of mitochondria from astrocytes to neurons after stroke. Nature 535(7613):551–555
Herguedas B, Watson JF, Ho H, Cais O, Garcia-Nafria J, Greger IH (2019) Architecture of the heteromeric GluA1/2 AMPA receptor in complex with the auxiliary subunit TARP gamma8. Science 364(6438):eaav9011. https://doi.org/10.1126/science.aav9011
Hong S, Li T, Luo Y, Li W, Tang X, Ye Y et al (2018) Dynamic changes of astrocytes and adenosine signaling in rat hippocampus in post-status epilepticus model of epileptogenesis. Cell Mol Neurobiol 38(6):1227–1234
Hossmann KA (2003) Glutamate hypothesis of stroke. Fortsch Neurol Psychiatr 71(Suppl 1):S10–S15
Jackson EK, Kotermanski SE, Menshikova EV, Dubey RK, Jackson TC, Kochanek PM (2017) Adenosine production by brain cells. J Neurochem 141(5):676–693
Kakegawa W, Miyoshi Y, Hamase K, Matsuda S, Matsuda K, Kohda K et al (2011) D-serine regulates cerebellar LTD and motor coordination through the delta2 glutamate receptor. Nat Neurosci 14(5):603–611
Kassubek R, Gorges M, Schocke M, Hagenston VAM, Huss A, Ludolph AC et al (2017) GFAP in early multiple sclerosis: a biomarker for inflammation. Neurosci Lett 657:166–170
Khatri R, McKinney AM, Swenson B, Janardhan V (2012) Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology 79(13 Suppl 1):S52–S57
Kitagawa H, Mori A, Shimada J, Mitsumoto Y, Kikuchi T (2002) Intracerebral adenosine infusion improves neurological outcome after transient focal ischemia in rats. Neurol Res 24(3):317–323
Kleopa KA, Sargiannidou I (2015) Connexins, gap junctions and peripheral neuropathy. Neurosci Lett 596:27–32
Laird DW (2006) Life cycle of connexins in health and disease. Biochem J 394(Pt 3):527–543
Landhuis E (2018) Tapping into the brain’s star power. Nature 563(7729):141–143
Lee Y, Messing A, Su M, Brenner M (2008) GFAP promoter elements required for region-specific and astrocyte-specific expression. Glia 56(5):481–493
Levy EI, Mokin M (2017) Stroke: Stroke thrombolysis and thrombectomy—not stronger together. Nat Rev Neurol 13(4):198–200
Li Q, Li QQ, Jia JN, Liu ZQ, Zhou HH, Mao XY (2019) Targeting gap junction in epilepsy: perspectives and challenges. Biomed Pharmacother 109:57–65. https://doi.org/10.1016/j.biopha.2018.10.068
Lin X, Xu Q, Veenstra RD (2014) Functional formation of heterotypic gap junction channels by connexins-40 and -43. Channels 8(5):433–443
Lin Z, Huang P, Huang S, Guo L, Xu X, Shen X et al (2018) Effect of adenosine and adenosine receptor antagonists on retinal Muller cell inwardly rectifying potassium channels under exogenous glutamate stimulation. Biomed Res Int 2018:2749257
Liu YJ, Chen J, Li X, Zhou X, Hu YM, Chu SF et al (2019) Research progress on adenosine in central nervous system diseases. CNS Neurosci Ther 25(9):899–910. https://doi.org/10.1111/cns.13190
Madji HB, Blasco H, Coque E, Vourc'h P, Emond P, Corcia P et al (2018) The metabolic disturbances of motoneurons exposed to glutamate. Mol Neurobiol 55(10):7669–7676
Matos M, Augusto E, Oliveira CR, Agostinho P (2008) Amyloid-beta peptide decreases glutamate uptake in cultured astrocytes: involvement of oxidative stress and mitogen-activated protein kinase cascades. Neuroscience 156(4):898–910
Matos M, Augusto E, Machado NJ, dos Santos-Rodrigues A, Cunha RA, Agostinho P (2012) Astrocytic adenosine A2A receptors control the amyloid-beta peptide-induced decrease of glutamate uptake. J Alzheimers Dis (JAD) 31(3):555–567
Matos M, Augusto E, Agostinho P, Cunha RA, Chen JF (2013) Antagonistic interaction between adenosine A2A receptors and Na+/K+-ATPase-alpha2 controlling glutamate uptake in astrocytes. J Neurosci 33(47):18492–18502
Matsuoka I, Ohkubo S (2004) ATP- and adenosine-mediated signaling in the central nervous system: adenosine receptor activation by ATP through rapid and localized generation of adenosine by ecto-nucleotidases. J Pharmacol Sci 94(2):95–99
Mayorquin LC, Rodriguez AV, Sutachan JJ, Albarracin SL (2018) Connexin-mediated functional and metabolic coupling between astrocytes and neurons. Front Mol Neurosci 11:118
Mazuel L, Schulte RF, Cladiere A, Speziale C, Lagree M, Leremboure M et al (2017) Intracerebral synthesis of glutamine from hyperpolarized glutamate. Magn Reson Med 78(4):1296–1305. https://doi.org/10.1002/mrm.26522
Melani A, Dettori I, Corti F, Cellai L, Pedata F (2015) Time-course of protection by the selective A2A receptor antagonist SCH58261 after transient focal cerebral ischemia. Neurol Sci 36(8):1441–1448. https://doi.org/10.1007/s10072-015-2160-y
Meretoja A, Acciarresi M, Akinyemi RO, Campbell B, Dowlatshahi D, English C et al (2017) Stroke doctors: who are we? A World Stroke Organization survey. Int J Stroke 12(8):858–868
Moidunny S, Vinet J, Wesseling E, Bijzet J, Shieh CH, van Ijzendoorn SC et al (2012) Adenosine A2B receptor-mediated leukemia inhibitory factor release from astrocytes protects cortical neurons against excitotoxicity. J Neuroinflammation 9:198
Oguro K, Jover T, Tanaka H, Lin Y, Kojima T, Oguro N et al (2001) Global ischemia-induced increases in the gap junctional proteins connexin 32 (Cx32) and Cx36 in hippocampus and enhanced vulnerability of Cx32 knock-out mice. J Neurosci 21(19):7534–7542
Okada M, Fukuyama K, Shiroyama T, Ueda Y (2019) Carbamazepine attenuates astroglial L-glutamate release induced by pro-inflammatory cytokines via chronically activation of adenosine A2A receptor. Int J Mol Sci 20(15):E3727. https://doi.org/10.3390/ijms20153727
Pintor A, Galluzzo M, Grieco R, Pezzola A, Reggio R, Popoli P (2004) Adenosine A 2A receptor antagonists prevent the increase in striatal glutamate levels induced by glutamate uptake inhibitors. J Neurochem 89(1):152–156
Radojkovic M, Stojanovic M, Stanojevic G, Radojkovic D, Gligorijevic J, Ilic I et al (2017) Ischemic preconditioning vs adenosine vs prostaglandin E1 for protection against liver ischemia/reperfusion injury. Braz J Med Biol Res 50(8):e6185. https://doi.org/10.1590/1414-431X20176185
Reiner A, Levitz J (2018) Glutamatergic signaling in the central nervous system: ionotropic and metabotropic receptors in concert. Neuron 98(6):1080–1098
Ribeiro FM, Vieira LB, Pires RG, Olmo RP, Ferguson SS (2017) Metabotropic glutamate receptors and neurodegenerative diseases. Pharmacol Res 115:179–191. https://doi.org/10.1016/j.phrs.2016.11.013
Rimmele TS, de Castro Abrantes H, Wellbourne-Wood J, Lengacher S, Chatton JY (2018) Extracellular potassium and glutamate interact to modulate mitochondria in astrocytes. ACS Chem Neurosci 9(8):2009–2015
Rimmele TS, Rosenberg PA (2016) GLT-1: The elusive presynaptic glutamate transporter. Neurochem Int 98:19–28
Robinson MB, Lee ML, DaSilva S (2020) Glutamate transporters and mitochondria: signaling, co-compartmentalization, functional coupling, and future directions. Neurochem Res 45(3):526–540. https://doi.org/10.1007/s11064-020-02974-8
Rose CR, Ziemens D, Untiet V, Fahlke C (2018) Molecular and cellular physiology of sodium-dependent glutamate transporters. Brain Res Bull 136:3–16
Schousboe A, Waagepetersen HS (2005) Role of astrocytes in glutamate homeostasis: implications for excitotoxicity. Neurotox Res 8(3–4):221–225
Sekiguchi KJ, Shekhtmeyster P, Merten K, Arena A, Cook D, Hoffman E et al (2016) Imaging large-scale cellular activity in spinal cord of freely behaving mice. Nat Commun 7:11450
Seydyousefi M, Moghanlou AE, Metz GAS, Gursoy R, Faghfoori MH, Mirghani SJ, Faghfoori Z (2019) Exogenous adenosine facilitates neuroprotection and functional recovery following cerebral ischemia in rats. Brain Res Bull 153:250–256
Shao W, Zhang SZ, Tang M, Zhang XH, Zhou Z, Yin YQ et al (2013) Suppression of neuroinflammation by astrocytic dopamine D2 receptors via alpha B-crystallin. Nature 494(7435):90–94
Sheikhbahaei S, Turovsky EA, Hosford PS, Hadjihambi A, Theparambil SM, Liu B et al (2018) Astrocytes modulate brainstem respiratory rhythm-generating circuits and determine exercise capacity. Nat Commun 9(1):370
Sheng M, Nakagawa T (2002) Neurobiology: glutamate receptors on the move. Nature 417(6889):601–602
Singh L, Kulshrestha R, Singh N, Jaggi AS (2018a) Mechanisms involved in adenosine pharmacological preconditioning-induced cardioprotection. Korean J Physiol Pharmacol 22(3):225–234
Singh L, Virdi JK, Maslov LN, Singh N, Jaggi AS (2018b) Investigating the possible mechanisms involved in adenosine preconditioning-induced cardioprotection in rats. Cardiovasc Ther 36(3):e12328
Singh H, Kumar M, Singh N, Jaggi AS (2019) Late phases of cardioprotection during remote ischemic preconditioning and adenosine preconditioning involve activation of neurogenic pathway. J Cardiovasc Pharmacol 73(2):63–69
Smail H, Baste JM, Gay A, Begueret H, Noel R, Morin JP et al (2016) Role of inflammatory cells and adenosine in lung ischemia reoxygenation injury using a model of lung donation after cardiac death. Exp Lung Res 42(3):131–141
Smith M, Reddy U, Robba C (2019) Acute ischaemic stroke: challenges for the intensivist. Intensive Care Med 45(9):1177–1189. https://doi.org/10.1007/s00134-019-05705-y
Solan JL, Lampe PD (2014) Specific Cx43 phosphorylation events regulate gap junction turnover in vivo. FEBS Lett 588(8):1423–1429
Sun K, Fan J, Han J (2015) Ameliorating effects of traditional Chinese medicine preparation, Chinese materia medica and active compounds on ischemia/reperfusion-induced cerebral microcirculatory disturbances and neuron damage. Acta Pharm Sin B 5(1):8–24
Thevenin AF, Margraf RA, Fisher CG, Kells-Andrews RM, Falk MM (2017) Phosphorylation regulates connexin43/ZO-1 binding and release, an important step in gap junction turnover. Mol Biol Cell 28(25):3595–3608
Tovar KR, Westbrook GL (2002) Mobile NMDA receptors at hippocampal synapses. Neuron 34(2):255–264
Trabelsi Y, Amri M, Becq H, Molinari F, Aniksztejn L (2017) The conversion of glutamate by glutamine synthase in neocortical astrocytes from juvenile rat is important to limit glutamate spillover and peri/extrasynaptic activation of NMDA receptors. Glia 65(2):401–415
Vicario N, Calabrese G, Zappala A, Parenti C, Forte S, Graziano ACE et al (2017) Inhibition of Cx43 mediates protective effects on hypoxic/reoxygenated human neuroblastoma cells. J Cell Mol Med 21(10):2563–2572
Wu LY, Yu XL, Feng LY (2015) Connexin 43 stabilizes astrocytes in a stroke-like milieu to facilitate neuronal recovery. Acta Pharmacol Sin 36(8):928–938
Yaghi S, Willey JZ, Cucchiara B, Goldstein JN, Gonzales NR, Khatri P et al (2017) Treatment and outcome of hemorrhagic transformation after intravenous alteplase in acute ischemic stroke: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 48(12):e343–e361. https://doi.org/10.1161/STR.0000000000000152
Yuan Q, Jia HX, Li SQ, Xiao-Zhang, Wu YJ, Feng L et al (2019) The role of adenosine in up-regulation of p38 MAPK and ERK during limb ischemic preconditioning-induced brain ischemic tolerance. Brain Res 1707:172–183
Zachariassen LG, Katchan L, Jensen AG, Pickering DS, Plested AJ, Kristensen AS et al (2016) Structural rearrangement of the intracellular domains during AMPA receptor activation. Proc Natl Acad Sci USA 113(27):E3950–E3959
Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S et al (2016) Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet 387(10015):251–272
Zhou Y, Zeng X, Li G, Yang Q, Xu J, Zhang M et al (2019) Inactivation of endothelial adenosine A2A receptors protects mice from cerebral ischaemia-induced brain injury. Brit J Pharmacol 176(13):2250–2263
Funding
This work was supported by the Hunan University of Chinese Medicine First-class Disciple Construction Project (201803), Hunan Engineering Technology Center of Standardization and Function of Chinese Herbal Decoction Pieces (2016TP2008), Hunan Provincial Natural Science Fund (S2019JJQNJJ1064), Project of NDRC and State Administration of Traditional Chinese Medicine (ZYBZH-Y-HUN-24).
Author information
Authors and Affiliations
Contributions
NC and YL involved in conceptualization and methodology; YL, YH, and SC participated in validation and writing the original draft; YL, XL, QZ, and QA did formal analysis; YL, SY, SR, HW, XX, and LG involved in writing, review, and editing of the manuscript. All authors approved the final submission.
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare no conflicts of interest.
Informed Consent
All participants in this study were informed and gave an informed written consent.
Research Involving Animals
All procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Animal Care and Use Committee of the Peking Union Medical College and Chinese Academy of Medical Sciences.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Y., Chu, S., Hu, Y. et al. Exogenous Adenosine Antagonizes Excitatory Amino Acid Toxicity in Primary Astrocytes. Cell Mol Neurobiol 41, 687–704 (2021). https://doi.org/10.1007/s10571-020-00876-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-020-00876-5