Abstract
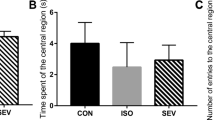
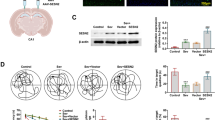
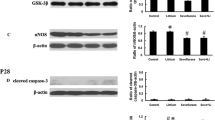
Sevoflurane is a widely used inhalational anesthetic in pediatric medicine that has been reported to have deleterious effects on the developing brain. Strategies to mitigate these detrimental effects are lacking. Sirtuin 2 (SIRT2) is a member of nicotinamide adenine dinucleotide (NAD+)-dependent protein deacetylases involved in a wide range of pathophysiological processes. SIRT2 inhibition has emerged as a promising treatment for an array of neurological disorders. However, the direct effects of SIRT2 on anesthesia-induced damage to the immature brain are unclear. Neonatal rats were exposed to 3% sevoflurane or 30% oxygen for 2 h daily with or without SIRT2 inhibitor AK7 pretreatment from postnatal day 7 (P7) to P9. One cohort of rats were euthanized 6, 12, and/or 24 h after the last gas exposure, and brain tissues were harvested for biochemical analysis and/or immunohistochemical examination. Cognitive functions were evaluated using the open field and Morris water maze tests on P25 and P28–32, respectively. SIRT2 was significantly up-regulated in neonatal rat hippocampus at 6 and 12 h post-anesthesia. Pretreatment with SIRT2 inhibitor AK7 reversed sevoflurane-induced hippocampus-dependent cognitive impairments. Furthermore, AK7 administration mitigated sevoflurane-induced neuroinflammation and microglial activation. Concomitantly, AK7 inhibited pro-inflammatory/M1-related markers and increased anti-inflammatory/M2-related markers in microglia. AK7 might prevent sevoflurane-induced neuroinflammation by switching microglia from the M1 to M2 phenotype. Downregulation of SIRT2 may be a novel therapeutic target for alleviating anesthesia-induced developmental neurotoxicity.





Similar content being viewed by others
Abbreviations
- NAD:
-
Nicotinamide adenine dinucleotide
- HDACs:
-
Histone deacetylases
- CNS:
-
Central nervous system
- PK:
-
Parkinson’s disease
- HD:
-
Huntington’s disease
- LPS:
-
Lipopolysaccharides
- MWM:
-
Morris water maze
- HATs:
-
Histone acetyltransferases
References
Biella G, Fusco F, Nardo E, Bernocchi O, Colombo A, Lichtenthaler SF, Forloni G, Albani D (2016) Sirtuin 2 inhibition improves cognitive performance and acts on amyloid-beta protein precursor processing in two Alzheimer’s disease mouse models. J Alzheimer’s Dis 53(3):1193–1207. https://doi.org/10.3233/jad-151135
Bilotta F, Evered LA, Gruenbaum SE (2017) Neurotoxicity of anesthetic drugs: an update. Curr Opin Anaesthesiol 30(4):452–457. https://doi.org/10.1097/aco.0000000000000482
Buechler N, Wang X, Yoza BK, McCall CE, Vachharajani V (2017) Sirtuin 2 regulates microvascular inflammation during sepsis. J Immunol Res 2017:2648946. https://doi.org/10.1155/2017/2648946
Chen H, Wu D, Ding X, Ying W (2015) SIRT2 is required for lipopolysaccharide-induced activation of BV2 microglia. NeuroReport 26(2):88–93. https://doi.org/10.1097/wnr.0000000000000305
Chopra V, Quinti L, Kim J, Vollor L, Narayanan KL, Edgerly C, Cipicchio PM, Lauver MA, Choi SH, Silverman RB, Ferrante RJ, Hersch S, Kazantsev AG (2012) The sirtuin 2 inhibitor AK-7 is neuroprotective in Huntington’s disease mouse models. Cell Rep 2(6):1492–1497. https://doi.org/10.1016/j.celrep.2012.11.001
Franco R, Fernandez-Suarez D (2015) Alternatively activated microglia and macrophages in the central nervous system. Prog Neurobiol 131:65–86. https://doi.org/10.1016/j.pneurobio.2015.05.003
Fujita Y, Yamashita T (2018) Sirtuins in neuroendocrine regulation and neurological diseases. Front Neurosci 12:778. https://doi.org/10.3389/fnins.2018.00778
Ganai SA, Banday S, Farooq Z, Altaf M (2016a) Modulating epigenetic HAT activity for reinstating acetylation homeostasis: a promising therapeutic strategy for neurological disorders. Pharmacol Ther 166:106–122. https://doi.org/10.1016/j.pharmthera.2016.07.001
Ganai SA, Ramadoss M, Mahadevan V (2016b) Histone deacetylase (HDAC) inhibitors: emerging roles in neuronal memory, learning, synaptic plasticity and neural regeneration. Curr Neuropharmacol 14(1):55–71
Gehrmann J, Matsumoto Y, Kreutzberg GW (1995) Microglia: intrinsic immuneffector cell of the brain. Brain Res Rev 20(3):269–287
Harrison IF, Smith AD, Dexter DT (2018) Pathological histone acetylation in Parkinson’s disease: neuroprotection and inhibition of microglial activation through SIRT 2 inhibition. Neurosci Lett 666:48–57. https://doi.org/10.1016/j.neulet.2017.12.037
Harting K, Knoll B (2010) SIRT2-mediated protein deacetylation: an emerging key regulator in brain physiology and pathology. Eur J Cell Biol 89(2–3):262–269. https://doi.org/10.1016/j.ejcb.2009.11.006
Jiao FZ, Wang Y, Zhang WB, Zhang HY, Chen Q, Shi CX, Wang LW, Gong ZJ (2019) Protective role of AGK2 on thioacetamide-induced acute liver failure in mice. Life Sci. https://doi.org/10.1016/j.lfs.2019.05.061
Jung YJ, Lee AS, Nguyen-Thanh T, Kim D, Kang KP, Lee S, Park SK, Kim W (2015) SIRT2 regulates LPS-induced renal tubular CXCL2 and CCL2 expression. J Am Soc Nephrol 26(7):1549–1560. https://doi.org/10.1681/asn.2014030226
Lee YM, Song BC, Yeum KJ (2015) Impact of volatile anesthetics on oxidative stress and inflammation. Biomed Res Int 2015:242709. https://doi.org/10.1155/2015/242709
Li Y, Shen R, Wen G, Ding R, Du A, Zhou J, Dong Z, Ren X, Yao H, Zhao R, Zhang G, Lu Y, Wu X (2017) Effects of ketamine on levels of inflammatory cytokines IL-6, IL-1beta, and TNF-alpha in the hippocampus of mice following acute or chronic administration. Front Pharmacol 8:139. https://doi.org/10.3389/fphar.2017.00139
Lin J, Sun B, Jiang C, Hong H, Zheng Y (2013) Sirt2 suppresses inflammatory responses in collagen-induced arthritis. Biochem Biophys Res Commun 441(4):897–903. https://doi.org/10.1016/j.bbrc.2013.10.153
Liu Y, Ao L, Li Y, Zhao Y, Wen Y, Ding H (2019) The SIRT2 inhibitor AK-7 decreases cochlear cell apoptosis and attenuates noise-induced hearing loss. Biochem Biophys Res Commun 509(3):641–646. https://doi.org/10.1016/j.bbrc.2018.12.084
Lo Sasso G, Menzies KJ, Mottis A, Piersigilli A, Perino A, Yamamoto H, Schoonjans K, Auwerx J (2014) SIRT2 deficiency modulates macrophage polarization and susceptibility to experimental colitis. PLoS ONE 9(7):e103573. https://doi.org/10.1371/journal.pone.0103573
Luthi-Carter R, Taylor DM, Pallos J, Lambert E, Amore A, Parker A, Moffitt H, Smith DL, Runne H, Gokce O, Kuhn A, Xiang Z, Maxwell MM, Reeves SA, Bates GP, Neri C, Thompson LM, Marsh JL, Kazantsev AG (2010) SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc Natl Acad Sci USA 107(17):7927–7932. https://doi.org/10.1073/pnas.1002924107
Maxwell MM, Tomkinson EM, Nobles J, Wizeman JW, Amore AM, Quinti L, Chopra V, Hersch SM, Kazantsev AG (2011) The Sirtuin 2 microtubule deacetylase is an abundant neuronal protein that accumulates in the aging CNS. Hum Mol Genet 20(20):3986–3996. https://doi.org/10.1093/hmg/ddr326
North BJ, Marshall BL, Borra MT, Denu JM, Verdin E (2003) The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell 11(2):437–444
Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ, Young AB, Abagyan R, Feany MB, Hyman BT, Kazantsev AG (2007) Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science 317(5837):516–519. https://doi.org/10.1126/science.1143780
Pais TF, Szego EM, Marques O, Miller-Fleming L, Antas P, Guerreiro P, de Oliveira RM, Kasapoglu B, Outeiro TF (2013) The NAD-dependent deacetylase sirtuin 2 is a suppressor of microglial activation and brain inflammation. EMBO J 32(19):2603–2616. https://doi.org/10.1038/emboj.2013.200
Pei Z, Wang S, Li Q (2017) Sevoflurane suppresses microglial M2 polarization. Neurosci Lett 655:160–165. https://doi.org/10.1016/j.neulet.2017.07.001
Penney J, Tsai LH (2014) Histone deacetylases in memory and cognition. Science signaling 7(355):re12. https://doi.org/10.1126/scisignal.aaa0069
Rothgiesser KM, Erener S, Waibel S, Luscher B, Hottiger MO (2010) SIRT2 regulates NF-kappaB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci 123(Pt 24):4251–4258. https://doi.org/10.1242/jcs.073783
Satomoto M, Makita K (2016) Anesthesia-induced neurotoxicity in an animal model of the developing brain: mechanism and therapies. Neural Regen Res 11(9):1407–1408. https://doi.org/10.4103/1673-5374.191207
Sen N (2015) Epigenetic regulation of memory by acetylation and methylation of chromatin: implications in neurological disorders, aging, and addiction. NeuroMol Med 17(2):97–110. https://doi.org/10.1007/s12017-014-8306-x
Shen X, Dong Y, Xu Z, Wang H, Miao C, Soriano SG, Sun D, Baxter MG, Zhang Y, Xie Z (2013) Selective anesthesia-induced neuroinflammation in developing mouse brain and cognitive impairment. Anesthesiology 118(3):502–515. https://doi.org/10.1097/ALN.0b013e3182834d77
Singh P, Hanson PS, Morris CM (2017) Sirtuin-2 protects neural cells from oxidative stress and is elevated in neurodegeneration. Parkinson’s Dis 2017:2643587. https://doi.org/10.1155/2017/2643587
Tang X, Chen XF, Wang NY, Wang XM, Liang ST, Zheng W, Lu YB, Zhao X, Hao DL, Zhang ZQ, Zou MH, Liu DP, Chen HZ (2017) SIRT2 acts as a cardioprotective deacetylase in pathological cardiac hypertrophy. Circulation 136(21):2051–2067. https://doi.org/10.1161/circulationaha.117.028728
Taylor DM, Balabadra U, Xiang Z, Woodman B, Meade S, Amore A, Maxwell MM, Reeves S, Bates GP, Luthi-Carter R, Lowden PA, Kazantsev AG (2011) A brain-permeable small molecule reduces neuronal cholesterol by inhibiting activity of sirtuin 2 deacetylase. ACS Chem Biol 6(6):540–546. https://doi.org/10.1021/cb100376q
Wang B, Zhang Y, Cao W, Wei X, Chen J, Ying W (2016) SIRT2 plays significant roles in lipopolysaccharides-induced neuroinflammation and brain injury in mice. Neurochem Res 41(9):2490–2500. https://doi.org/10.1007/s11064-016-1981-2
Wang J, Koh HW, Zhou L, Bae UJ, Lee HS, Bang IH, Ka SO, Oh SH, Bae EJ, Park BH (2017) Sirtuin 2 aggravates postischemic liver injury by deacetylating mitogen-activated protein kinase phosphatase-1. Hepatology 65(1):225–236. https://doi.org/10.1002/hep.28777
Whitaker EE, Christofi FL, Quinn KM, Wiemann BZ, Xia JC, Tobias JD, Bissonnette B (2017) Selective induction of IL-1beta after a brief isoflurane anesthetic in children undergoing MRI examination. J Anesth 31(2):219–224. https://doi.org/10.1007/s00540-016-2294-y
Wu Z, Li X, Zhang Y, Tong D, Wang L, Zhao P (2018) Effects of sevoflurane exposure during mid-pregnancy on learning and memory in offspring rats: beneficial effects of maternal exercise. Front Cell Neurosci 12:122. https://doi.org/10.3389/fncel.2018.00122
Yuan F, Xu ZM, Lu LY, Nie H, Ding J, Ying WH, Tian HL (2016) SIRT2 inhibition exacerbates neuroinflammation and blood-brain barrier disruption in experimental traumatic brain injury by enhancing NF-kappaB p65 acetylation and activation. J Neurochem 136(3):581–593. https://doi.org/10.1111/jnc.13423
Zhang MQ, Ji MH, Zhao QS, Jia M, Qiu LL, Yang JJ, Peng YG, Yang JJ, Martynyuk AE (2015) Neurobehavioural abnormalities induced by repeated exposure of neonatal rats to sevoflurane can be aggravated by social isolation and enrichment deprivation initiated after exposure to the anaesthetic. Br J Anaesth 115(5):752–760. https://doi.org/10.1093/bja/aev339
Zhao T, Alam HB, Liu B, Bronson RT, Nikolian VC, Wu E, Chong W, Li Y (2015) Selective inhibition of SIRT2 improves outcomes in a lethal septic model. Curr Mol Med 15(7):634–641
Funding
This work was supported by the National Nature Science Foundation of China (Nos. 81870838, 81671311), the Key Research and Development Program of Liaoning Province (No. 2018225004), and the Outstanding Scientific Fund of Shengjing Hospital (No. 201708).
Author information
Authors and Affiliations
Contributions
ZW and PZ conceived and designed experiments. ZW, YZ (Yi Zhang) and YZ (Yinong Zhang) performed experiments, generated and analyzed the data. ZW wrote the manuscript with the help of PZ. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the Animal Ethics Committee of Shengjing Hospital of China Medical University, where these studies were conducted. This article does not contain any studies with human participants performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.

Supplementary Fig.
1 Expressions of acetylated α-tubulin in the hippocampus of rats in each group. (a) Representative western blotting images for the expression levels of acetylated α-tubulin. (b) Quantification of acetylated α-tubulin normalized to α-tubulin. n = 5 per group. Data are expressed as mean ± SD of control mean values. One-way ANOVA followed by Tukey post hoc multiple comparison tests was used for data analysis. **P < 0.01, compared with CON group; ##P < 0.01, compared with SEV group. Supplementary material 1 (JPEG 172 kb)
Rights and permissions
About this article
Cite this article
Wu, Z., Zhang, Y., Zhang, Y. et al. Sirtuin 2 Inhibition Attenuates Sevoflurane-Induced Learning and Memory Deficits in Developing Rats via Modulating Microglial Activation. Cell Mol Neurobiol 40, 437–446 (2020). https://doi.org/10.1007/s10571-019-00746-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-019-00746-9