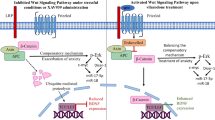
Abstract
Exposure to corticosterone attenuates hippocampal CA1 long-term potentiation (LTP) via intracellular Zn2+ dysregulation. Here we report that effusol, a phenanthrene isolated from Chinese medicine Juncus effusus, rescues CA1 LTP attenuated by corticosterone. In vivo microdialysis experiment indicated that both increases in extracellular glutamate induced under perfusion with corticosterone and high K+ are suppressed in the hippocampus by co-perfusion with effusol. Because corticosterone and high K+ also increase extracellular Zn2+ level, followed by intracellular Zn2+ dysregulation, the effect of effusol on both the increases was examined in brain slice experiments. Effusol did not suppress increase in extracellular Zn2+ in the hippocampal CA1 of brain slices bathed in corticosterone, but suppressed increase in intracellular Zn2+, which may be linked with suppressing the increase in extracellular glutamate in vivo. In vivo CA1 LTP was attenuated under perfusion with corticosterone prior to LTP induction, while the attenuation was rescued by co-perfusion with effusol, suggesting that the rescuing effect of effusol is due to suppressing the increase in intracellular Zn2+ in CA1 pyramidal cells. The present study indicates that CA1 LTP attenuated by corticosterone is canceled by effusol, which rescues intracellular Zn2+ dysregulation via suppressing extracellular glutamate accumulation. It is likely that effusol defends the hippocampal function against stress-induced cognitive decline.








Similar content being viewed by others
Abbreviations
- LTP:
-
Long-term potentiation
- MC:
-
Mineralocorticoid
- GC:
-
Glucocorticoid
- CS:
-
Corticosterone
- AMPA:
-
α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionate
- GABA:
-
γ-Aminobutyric acid
- ACSF:
-
Artificial cerebrospinal fluid
- PCL:
-
Pyramidal cell layer
- SR:
-
Stratum radiatum
References
Dorey R, Piérard C, Shinkaruk S, Tronche C, Chauveau F, Baudonnat M, Béracochéa D (2011) Membrane mineralocorticoid but not glucocorticoid receptors of the dorsal hippocampus mediate the rapid effects of corticosterone on memory retrieval. Neuropsychopharmacology 36:2639–2649
Frederickson CJ, Koh JY, Bush AI (2005) The neurobiology of zinc in health and disease. Nat Rev Neurosci 6:449–462
Greca MD, Fiorentino A, Molinaro A, Monaco P, Previtera L (1993) A bioactive dihydrodibenzoxepin from Juncus effusus. Phytochemistry 34:1182–1184
Greca MD, Fiorention A, Monaco P, Previtera L (1994) Cycloartane triterpenes from Juncus effusus. Phytochemistry 35:1017–1022
Greca MD, Fiorentino A, Monaco P, Pinto G, Pollio A, Previtera L (1996) Action of antialgal compounds from Juncus effusus L. on Selenastrum capricornutum. J Chem Ecol 22:587–603
Hirano T, Kikuchi K, Urano Y, Nagano T (2002) Improvement and biological applications of fluorescent probes for zinc, ZnAFs. J Am Chem Soc 124:6555–6562
Joëls M, de Kloet ER (2017) The brain mineralocorticoid receptor: a saga in three episodes. J Endocrinol 234:T49–T66
Joëls M, Karst H, DeRijk R, de Kloet ER (2008) The coming out of the brain mineralocorticoid receptor. Trends Neurosci 31:1–7
Karst H, Berger S, Turiault M, Tronche F, Schutz G, Joels M (2005) Mineralocorticoid receptors are indispensable for nongenomic modulation of hippocampal glutamate transmission by corticosterone. Proc Natl Acad Sci USA 102:19204–19207
Khaksari M, Rashidy-Pour A, Vafaei AA (2007) Central mineralocorticoid receptors are indispensable for corticosterone-induced impairment of memory retrieval in rats. Neuroscience 149:729–738
Kim JJ, Diamond DM (2002) The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci 3:453–462
Kim J, Yoon KS (1998) Stress: metaplastic effects in the hippocampus. Trends Neurosci 21:505–509
Kwak S, Weiss JH (2006) Calcium-permeable AMPA channels in neurodegenerative disease and ischemia. Curr Opin Neurobiol 16:281–287
Liao YJ, Zhai HF, Zhang B, Duan TX, Huang JM (2011) Anxiolytic and sedative effects of dehydroeffusol from Juncus effusus in mice. Planta Med 77:416–420
Lynch MA (2004) Long-term potentiation and memory. Physiol Rev 84:87–136
McEwen BS, Sapolsky RM (1995) Stress and cognitive function. Curr Opin Neurobiol 5:205–216
McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C (2015) Mechanisms of stress in the brain. Nat Neurosci 18:1353–1363
Olijslagers JE, De Kloet ER, Elgersma Y, Van Woerden GM, Joëls M, Karst K (2008) Rapid changes in hippocampal CA1 pyramidal cell function via pre- as well as postsynaptic membrane mineralocorticoid receptors. Eur J Neurosci 27:2542–2550
Sajadi AA, Samaei SA, Rashidy-Pour A (2006) Intra-hippocampal microinjections of anisomycin did not block glucocorticoid-induced impairment of memory retrieval in rats: an evidence for non-genomic effects of glucocorticoids. Behav Brain Res 173:158–162
Sandi C (2011) Glucocorticoids act on glutamatergic pathways to affect memory processes. Trends Neurosci 34:165–176
Shima K, Toyota M, Asakawa Y (1991) Phenanthrene derivatives from the medullae of Juncus effusus. Phytochemistry 30:3149–3151
Sindreu CB, Varoqui H, Erickson JD, Perez-Clausell J (2003) Boutons containing vesicular zinc define a subpopulation of synapses with low AMPAR content in rat hippocampus. Cereb Cortex 13:823–829
Singhuber J, Baburin I, Khom S, Zehl M, Urban E, Hering S, Kopp B (2012) GABA(A) receptor modulators from the Chinese herbal drug Junci Medulla—the pith of Juncus effusus. Planta Med 78:455–458
Suh SW, Jo SM, Vajda Z, Danscher G (2001) Adrenalectomy causes loss of zinc ions in zinc-enriched (ZEN) terminals and decreases seizure-induced neuronal death. Brain Res 895:25–32
Suzuki M, Sato Y, Tamura K, Tamano H, Takeda A (2018) Rapid intracellular Zn2+ dysregulation via membrane corticosteroid receptor activation affects in vivo CA1 LTP. Mol Neurobiol. https://doi.org/10.1007/s12035-018-1159-9
Takeda A, Fuke S, Tsutsumi W, Oku N (2007) Negative modulation of presynaptic activity by zinc released from Schaffer collaterals. J Neurosci Res 85:3666–3672
Takeda A, Ando M, Kanno S, Oku N (2009) Unique response of zinc in the hippocampus to behavioral stress and attenuation of subsequent mossy fiber long-term potentiation. NeuroToxicology 30:712–717
Takeda A, Takada S, Nakamura M, Suzuki M, Tamano H, Ando M, Oku N (2011) Transient increase in Zn2+ in hippocampal CA1 pyramidal neurons causes reversible memory deficit. PLoS ONE 6:e28615
Takeda A, Suzuki M, Tamano H, Takada S, Ide K, Oku K (2012) Involvement of glucocorticoid-mediated Zn2+ signaling in attenuation of hippocampal CA1 LTP by acute stress. Neurochem Int 60:394–399
Takeda A, Koike Y, Osawa M, Tamano H (2018a) Characteristic of extracellular Zn2+ influx in the middle-aged dentate gyrus and its involvement in attenuation of LTP. Mol Neurobiol 55:2185–2195
Takeda A, Tamano H, Hisatsune M, Murakami T, Nakada H, Fujii H (2018b) Maintained LTP and memory are lost by Zn2+ influx into dentate granule cells, but not Ca2+ influx. Mol Neurobiol 55:1498–1508
Wang Y, Wang Y, Zhai H, Liao Y, Zhang B, Huang J (2012) Phenanthrenes from Juncus effusus with anxiolytic and sedative activities. Nat Prod Res 26:1234–1239
Weiss JH (2011) Ca permeable AMPA channels in diseases of the nervous system. Front Mol Neurosci 4:42
Author information
Authors and Affiliations
Contributions
Planned experiments: H.T. and A.T. Performed the Experiments: Y.S., T.M, and T.F. Analyzed data: H.K. and M.S. Wrote the paper: H.T. and A.T. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors have no conflict of interest.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tamano, H., Sato, Y., Takiguchi, M. et al. CA1 LTP Attenuated by Corticosterone is Canceled by Effusol via Rescuing Intracellular Zn2+ Dysregulation. Cell Mol Neurobiol 39, 975–983 (2019). https://doi.org/10.1007/s10571-019-00693-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-019-00693-5