Abstract
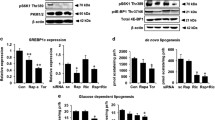
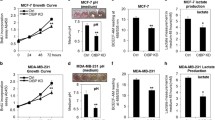
Glucose and glutamine are two essential ingredients for cell growth. Glycolysis and glutaminolysis can be linked by glutamine: fructose-6-phosphate aminotransferase (GFAT, composed of GFAT1 and GFAT2) that catalyzes the synthesis of glucosamine-6-phosphate and glutamate by using fructose-6-phosphate and glutamine as substrates. The role of mammalian target of rapamycin (MTOR, composed of MTOR1 and MTOR2) in regulating glycolysis has been explored in human cancer cells. However, whether MTOR can interact with GFAT to regulate glucosamine-6-phosphate is poorly understood. In this study, we report that GFAT1 is essential to maintain the malignant features of GBM cells. And MTOR2 rather than MTOR1 plays a robust role in promoting GFAT1 protein activity, and accelerating the progression of glucosamine-6-phosphate synthesis, which is not controlled by the PI3K/AKT signaling. Intriguingly, high level of glucose or glutamine supply promotes MTOR2 protein activity. In turn, up-regulating glycolytic and glutaminolytic metabolisms block MTOR dimerization, enhancing the release of MTOR2 from the MTOR complex. As a transcriptional factor, C-MYC, directly targeted by MTOR2, promotes the relative mRNA expression level of GFAT1. Notably, our data reveal that GFAT1 immunoreactivity is positively correlated with the malignant grades of glioma patients. Kaplan–Meier assay reveals the correlations between patients’ 5-year survival and high GFAT1 protein expression. Taken together, we propose that the MTOR2/C-MYC/GFAT1 axis is responsible for the modulation on the crosstalk between glycolysis and glutaminolysis in GBM cells. Under the condition of accelerated glycolytic and/or glutaminolytic metabolisms, the MTOR2/C-MYC/GFAT1 axis will be up-regulated in GBM cells.










Similar content being viewed by others
Change history
07 June 2019
A correction has been published.
14 August 2019
The original version of this article unfortunately contained an error in author group. The authors Yi-Xiang See, Xin Chen, Zi-Kai Chen and Ze-Bin Huang were inadvertently included in the article.
References
Altman BJ, Stine ZE, Dang CV (2016) From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer 16:619–634
Biggs III WH, Meisenhelder J, Hunter T, Cavenee WK, Arden KC (1999) Protein kinase B/Akt-mediated phosphorylation promotes nuclear exclusion of the winged helix transcription factor FKHR1. Proc Natl Acad Sci USA 96:7421–7426
Cairns RA, Harris I, McCracken S, Mak TW (2011a) Cancer cell metabolism. Cold Spring Harb Symp Quant Biol 76:299–311
Cairns RA, Harris IS, Mak TW (2011b) Regulation of cancer cell metabolism. Nat Rev Cancer 11:85–95
Cantor JR, Sabatini DM (2012) Cancer cell metabolism: one hallmark, many faces. Cancer Discov 2:881–898
Choe G, Horvath S, Cloughesy TF, Crosby K, Seligson D, Palotie A, Inge L, Smith BL, Sawyers CL, Mischel PS (2003) Analysis of the phosphatidylinositol 30-kinase signaling pathway in GBM patients in vivo. Cancer Res 63:2742–2746
Christofk HR, Vander Heiden MG, Wu N, Asara JM, Cantley LC (2008) Pyruvate kinase M2 is a phosphotyrosine-binding protein. Nature 452:181–186
Dang CV (2012) Links between metabolism and cancer. Genes Dev 26:877–890
Dang CV, Le A, Gao P (2009) MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res 15:6479–6483
DeBerardinis RJ, Cheng T (2010) Q’s next: the diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 29:313–324
DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S et al (2007) Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA 104:19345–19350
DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB (2008) The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab 7:11–20
DeHaven JE, Robinson KA, Nelson BA, Buse MG (2001) A novel variant of glutamine: fructose-6-phosphate amidotransferase-1 (GFAT1) mRNA is selectively expressed in striated muscle. Diabetes 50:2419–2424
Faubert B, Boily G, Izreig S, Griss T, Samborska B, Dong Z, Dupuy F, Chambers C, Fuerth BJ, Viollet B et al (2013) AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab 17:113–124
Gan B, Lim C, Chu G, Hua S, Ding Z, Collins M, Hu J, Jiang S, Fletcher-Sananikone E, Zhuang L et al (2010) FoxOs enforce a progression checkpoint to constrain MTORC1-activated renal tumorigenesis. Cancer Cell 18:472–484
Gao P, Tchernyshyov I, Chang TC et al (2009) C-MYC suppression of miR23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458:762–765
Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM (2006) Ablation in mice of the MTORC components raptor, rictor, or mLST8 reveals that MTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell 11:859–871
Hagiwara A, Cornu M, Cybulski N, Polak P, Betz C, Trapani F, Terracciano L, Heim MH, Rüegg MA, Hall MN (2012) Hepatic MTORC2 activates glycolysis and lipogenesis through Akt, glucokinase, and SREBP1c. Cell Metab 15:725–738
Hosios AM, Hecht VC, Danai LV, Johnson MO, Rathmell JC, Steinhauser ML et al (2016) Amino acids rather than glucose account for the majority of cell mass in proliferating mammalian cells. Dev Cell 36:540–549
Hu Y, Riesland L, Paterson AJ, Kudlow JE (2004) Phosphorylation of mouse glutamine-fructose-6-phosphate amidotransferase 2 (GFAT2) by cAMP-dependent protein kinase increases the enzyme activity. J Biol Chem 279:29988–29993
Hudson CC, Liu M, Chiang GG, Otterness DM, Loomis DC, Kaper F, Giaccia AJ, Abraham RT (2002) Regulation of hypoxia-inducible factor 1alpha expression and function by the mammalian target of rapamycin. Mol Cell Biol 22:7004–7014
Kaelin WG Jr, Ratcliffe PJ (2008) Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell 30:393–402
Kleszcz R, Paluszczak J, Krajka-Kuźniak V, Baer-Dubowska W (2018) The inhibition of C-MYC transcription factor modulates the expression of glycolytic and glutaminolytic enzymes in FaDu hypopharyngeal carcinoma cells. Adv Clin Exp Med 27:735–742
Koppenol WH, Bounds PL, Dang CV (2011) Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer 11:325–337
Le A, Lane AN, Hamaker M, Bose S, Gouw A, Barbi J et al (2012) Glucose-independent glutamine metabolism via TCA cycling for proliferation and survival in B cells. Cell Metab 15:110–121
Lee M, Theodoropoulou M, Graw J, Roncaroli F, Zatelli MC, Pellegata NS (2011) Levels of p27 sensitize to dual PI3K/mTOR inhibition. Mol Cancer Ther 10:1450–1459
Levine AJ, Puzio-Kuter AM (2010) The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 330:134001344
Lunt SY, Vander Heiden MG (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu Rev Cell Dev Biol 27:441–464
Marroquin LD, Hynes J, Dykens JA, Jamieson JD, Will Y (2007) Circumventing the Crabtree effect: replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol Sci 97:539–547
Matsui Y, Lai ZC (2019) Bimolecular fluorescence complementation (BiFC) in tissue culture and in developing tissues of Drosophila to study protein–protein interactions. Methods Mol Biol 1893:75–85
Medina MA, Núñez de Castro I (1990) Glutaminolysis and glycolysis interactions in proliferant cells. Int J Biochem 22:681–683
Muellner MK, Uras IZ, Gapp BV, Kerzendorfer C, Smida M, Lechtermann H, Craig-Mueller N, Colinge J, Duernberger G, Nijman SM (2011) A chemical-genetic screen reveals a mechanism of resistance to PI3K inhibitors in cancer. Nat Chem Biol 7:787–793
Niimi M, Ogawara T, Yamashita T, Yamamoto Y, Ueyama A, Kambe T et al (2001) Identification of GFAT1-L, a novel splice variant of human glutamine: fructose-6-phosphate amidotransferase (GFAT1) that is expressed abundantly in skeletal muscle. J Hum Genet 46:566–571
Oki T, Yamazaki K, Kuromitsu J, Okada M, Tanaka I (1999) cDNA cloning and mapping of a novel subtype of glutamine:fructose-6-phosphate amidotransferase (GFAT2) in human and mouse. Genomics 57:227–234
Pelicano H, Martin DS, Xu RH, Huang P (2006) Glycolysis inhibition for anticancer treatment. Oncogene 25:4633–4646
Plas DR, Thompson CB (2005) Akt-dependent transformation: there is more to growth than just surviving. Oncogene 24:7435–7442
Qie S, Liang D, Yin C, Gu W, Meng M, Wang C et al (2012) Glutamine depletion and glucose depletion trigger growth inhibition via distinctive gene expression reprogramming. Cell Cycle 11:3679–3690
Rohle D, Popovici-Muller J, Palaskas N, Turcan S, Grommes C, Campos C, Tsoi J, Clark O, Oldrini B, Komisopoulou E et al (2013) An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 340:626–630
Shanware NP, Mullen AR, DeBerardinis RJ, Abraham RT (2011) Glutamine: pleiotropic roles in tumor growth and stress resistance. J Mol Med (Berl) 89:229–236
Tanaka K, Babic I, Nathanson D, Akhavan D, Guo D, Gini B, Dang J, Zhu S, Yang H, De Jesus J et al (2011) Oncogenic EGFR signaling activates an MTORC2-NF-kB pathway that promotes chemotherapy resistance. Cancer Discov 1:524–538
Tong X, Zhao F, Thompson CB (2009) The molecular determinants of de novo nucleotide biosynthesis in cancer cells. Curr Opin Genet Dev 19:32–37
Vadla R, Haldar D (2018) Mammalian target of rapamycin complex 2 (MTORC2) controls glycolytic gene expression by regulating Histone H3 Lysine 56 acetylation. Cell Cycle 17:110–123
Vander Heiden MG, Cantley LC, Thompson CB (2009) Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324:1029–1033
Vyas B, Silakari O, Bahia MS, Singh B (2013) Glutamine: fructose-6-phosphate amidotransferase (GFAT): homology modelling and designing of new inhibitors using pharmacophore and docking based hierarchical virtual screening protocol. SAR QSAR Environ Res 24:733–752
Wang B, Moya N, Niessen S, Hoover H, Mihaylova MM, Shaw RJ, Yates JR III, Fischer WH, Thomas JB, Montminy M (2011) A hormone-dependent module regulating energy balance. Cell 145:596–606
Wang QS, Kong PZ, Li XQ, Yang F, Feng YM (2015) FOXF2 deficiency promotes epithelial-mesenchymal transition and metastasis of basal-like breast cancer. Breast Cancer Res 17:30
Warburg O (1956) On the origin of cancer cells. Science 123:309–314
Ward PS, Thompson CB (2012) Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 21:297–308
Wellen KE, Lu C, Mancuso A, Lemons JM, Ryczko M, Dennis JW et al (2010) The hexosamine biosynthetic pathway couples growth factor-induced glutamine uptake to glucose metabolism. Genes Dev 24:2784–2799
Wise DR, DeBerardinis RJ, Mancuso A et al (2008) Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA 105:18782–18787
Wise DR, Thompson CB (2010) Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci 35:427–433
Yang C, Peng P, Li L, Shao M, Zhao J, Wang L, Duan F, Song S, Wu H, Zhang J, Zhao R, Jia D, Zhang M, Wu W, Li C, Rong Y, Zhang L, Ruan Y, Gu J (2016) High expression of GFAT1 predicts poor prognosis in patients with pancreatic cancer. Sci Rep 6:39044
Acknowledgements
We thank Dr. Qi Zhang (Tiantan Hospital, Beijing, China) for kindly collecting glioma tissue samples and clinical information. This research was funded by the Postdoctoral Research Foundation (2014) provided by the University of Macau, SAR, China.
Author information
Authors and Affiliations
Contributions
B.L. designed the research project, performed most of the experiments, analyzed the data, and wrote the manuscript. Z.B.H, X.C., Y.X.S., Z.K.C., and H.K.Y. performed the experiments and analyzed the data.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
This study used human glioma tissues and brain injury tissues obtained from Tiantan Hospital (Beijing, China). All procedures performed in studies involving human participants were in accordance with the Ethical Standards of the Institutional and/or National Research Committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, B., Huang, ZB., Chen, X. et al. Mammalian Target of Rapamycin 2 (MTOR2) and C-MYC Modulate Glucosamine-6-Phosphate Synthesis in Glioblastoma (GBM) Cells Through Glutamine: Fructose-6-Phosphate Aminotransferase 1 (GFAT1). Cell Mol Neurobiol 39, 415–434 (2019). https://doi.org/10.1007/s10571-019-00659-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-019-00659-7