Abstract
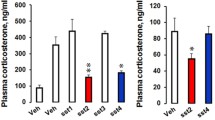
Mice lacking the substance P (SP) neurokinin-1 (NK1) receptor (NK1R−/−mice) were used to investigate whether SP affects serotonin (5-HT) function in the brain and to assess the effects of acute immobilisation stress on the hypothalamic–pituitary–adrenocortical (HPA) axis and 5-HT turnover in individual brain nuclei. Basal HPA activity and the expression of hypothalamic corticotropin-releasing hormone (CRH) in wild-type (WT)- and NK1R−/− mice were identical. Stress-induced increases in plasma ACTH concentration were considerably higher in NK1R−/− mice than in WT mice while corticosterone concentrations were equally elevated in both mouse lines. Acute stress did not alter the expression of CRH. In the dorsal raphe nucleus (DRN), basal 5-HT turnover was increased in NK1R−/− mice and a 15 min stress further magnified 5-HT utilisation in this region. In the frontoparietal cortex, medial prefrontal cortex, central nucleus of amygdala, and the hippocampal CA1 region, stress increased 5-HT and/or 5-hydroxyindoleacetic acid (5-HIAA) concentrations to a similar extent in WT and NK1R−/− mice. 5-HT turnover in the hypothalamic paraventricular nucleus was not affected by stress, but stress induced similar increases in 5-HT and 5-HIAA in the ventromedial and dorsomedial hypothalamic nuclei in WT and NK1R−/− mice. Our findings indicate that NK1 receptor activation suppresses ACTH release during acute stress but does not exert sustained inhibition of the HPA axis. Genetic deletion of the NK1 receptor accelerates 5-HT turnover in DRN under basal and stress conditions. No differences between the responses of serotonergic system to acute stress in WT and NK1R−/− mice occur in forebrain nuclei linked to the regulation of anxiety and neuroendocrine stress responses.


Similar content being viewed by others
References
Aguilera G, Liu Y (2012) The molecular physiology of CRH neurons. Front Neuroendocrinol 33:67–84. https://doi.org/10.1016/j.yfrne.2011.08.002
Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB (1999) The role of corticotropin-releasing factor in depression and anxiety disorders. J Endocrinol 160:1–12
Bernardis LL, Bellinger LL (1998) The dorsomedial hypothalamic nucleus revisited: 1998 update. Proc Soc Exp Biol Med 218:284–306
Binder EB, Nemeroff CB (2010) The CRF system, stress, depression and anxiety-insights from human genetic studies. Mol Psychiatry 15:574–588. https://doi.org/10.1038/mp.2009.141
Blier P, Gobbi G, Haddjeri N, Santarelli L, Mathew G, Hen R (2004) Impact of substance P receptor antagonism on the serotonin and norepinephrine systems: relevance to the antidepressant/anxiolytic response. J Psychiatry Neurosci 29:208–218
Canteras NS, Simerly RB, Swanson LW (1994) Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 348:41–79
Carrasco GA, Van de Kar LD (2003) Neuroendocrine pharmacology of stress. Eur J Pharmacol 463:235–272
Chaouloff F (1993)) Physiopharmacological interactions between stress hormones and central serotonergic systems. Brain Res Brain Res Rev 18:1–32
Chenu F, Guiard BP, Bourin M, Gardier AM (2006) Antidepressant-like activity of selective serotonin reuptake inhibitors combined with a NK1 receptor antagonist in the mouse forced swimming test. Behav Brain Res 172:256–263
Chiueh CC, Zukowska-Grojec Z, Kirk KL, Kopin IJ (1983) 6-Fluorocatecholamines as false adrenergic neurotransmitters. J Pharmacol Exp Ther 225:529–533
Commons KG, Connolley KR, Valentino RJ (2003) A neurochemically distinct dorsal raphe-limbic circuit with a potential role in affective disorders. Neuropsychopharmacology 28:206–215
Conley RK, Cumberbatch MJ, Mason GS, Williamson DJ, Harrison T, Locker K, Swain C, Maubach K, O’Donnell R, Rigby M, Hewson L, Smith D, Rupniak NMJ (2002) Substance P (neurokinin 1) receptor antagonists enhance dorsal raphe neuronal activity. J Neurosci 22:7730–7736
Cullinan WE, Ziegler DR, Herman JP (2008) Functional role of local GABAergic influences on the HPA axis. Brain Struct Funct 213:63–72. https://doi.org/10.1007/s00429-008-0192-2
Culman J, Kvetnanský R, Torda T, Murgas K (1980) Serotonin concentration in individual hypothalamic nuclei of rats exposed to acute immobilization stress. Neuroscience 5:1503–1506
Culman J, Kiss A, Kvetnanský R (1984) Serotonin and tryptophan hydroxylase in isolated hypothalamic and brain stem nuclei of rats exposed to acute and repeated immobilization stress. Exp Clin Endocrinol 83:28–36
Culman J, Das G, Ohlendorf C, Haass M, Maser-Gluth C, Zuhayra M, Zhao Y, Itoi K (2010) Blockade of tachykinin NK1/NK2 receptors in the brain attenuates the activation of corticotrophin-releasing hormone neurones in the hypothalamic paraventricular nucleus and the sympathoadrenal and pituitary-adrenal responses to formalin-induced pain in the rat. J Neuroendocrinol 22:467–476. https://doi.org/10.1111/j.1365-2826.2010.01987.x
Dalley JW, Cardinal RN, Robbins TW (2004) Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev 28:771–784
David DJ, Froger N, Guiard B, Przybylski C, Jego G, Boni C, Hunt SP, De Felipe C, Hamon M, Jacquot C, Gardier AM, Lanfumey L (2004) Serotonin transporter in substance P (neurokinin 1) receptor knock-out mice. Eur J Pharmacol 492:41–48
De Felipe C, Herrero JF, O’Brien JA, Palmer JA, Doyle CA, Smith AJ, Laird JM, Belmonte C, Cervero F, Hunt SP (1998) Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature 392:394–397
Delgado-Morales R, del Río E, Gómez-Román A, Bisagno V, Nadal R, de Felipe C, Armario A (2012) Adrenocortical and behavioural response to chronic restraint stress in neurokinin-1 receptor knockout mice. Physiol Behav 105:669–675. https://doi.org/10.1016/j.physbeh.2011.10.008
Ebner K, Muigg P, Singewald G, Singewald N (2008) Substance P in stress and anxiety: NK-1 receptor antagonism interacts with key brain areas of the stress circuitry. Ann N Y Acad Sci 1144:61–73. https://doi.org/10.1196/annals.1418.018
Feetham CH, Barrett-Jolley R (2014) NK1-receptor-expressing paraventricular nucleus neurones modulate daily variation in heart rate and stress-induced changes in heart rate variability. Physiol Rep. https://doi.org/10.14814/phy2.12207
Froger N, Gardier AM, Moratalla R, Alberti I, Lena I, Boni C, De Felipe C, Rupniak NM, Hunt SP, Jacquot C, Hamon M, Lanfumey L (2001) 5-hydroxytryptamine (5-HT)1A autoreceptor adaptive changes in substance P (neurokinin 1) receptor knock-out mice mimic antidepressant-induced desensitization. J Neurosci 21:8188–8197
Gobert A, Brocco M, Dekeyne A, Di Cara B, Bouchez G, Lejeune F, Gannon RL, Millan MJ (2009) Neurokinin1 antagonists potentiate antidepressant properties of serotonin reuptake inhibitors, yet blunt their anxiogenic actions: a neurochemical, electrophysiological, and behavioral characterization. Neuropsychopharmacology 34:1039–1056. https://doi.org/10.1038/npp.2008.176
Goldstein LE, Rasmusson AM, Bunney BS, Roth RH (1996) Role of the amygdala in the coordination of behavioral, neuroendocrine, and prefrontal cortical monoamine responses to psychological stress in the rat. J Neurosci 16:4787–4798
Guiard BP, Przybylski C, Guilloux JP, Seif I, Froger N, De Felipe C, Hunt SP, Lanfumey L, Gardier AM (2004) Blockade of substance P (neurokinin 1) receptors enhances extracellular serotonin when combined with a selective serotonin reuptake inhibitor: an in vivo microdialysis study in mice. J Neurochem 89:54–63
Guiard BP, Froger N, Hamon M, Gardier AM, Lanfumey L (2005) Sustained pharmacological blockade of NK1 substance P receptors causes functional desensitization of dorsal raphe 5-HT 1A autoreceptors in mice. J Neurochem 95:1713–1723
Guiard BP, Lanfumey L, Gardier AM (2006) Microdialysis approach to study serotonin outflow in mice following selective serotonin reuptake inhibitors and substance P (neurokinin 1) receptor antagonist administration: a review. Curr Drug Targets 7:187–201
Haddjeri N, Blier P (2001) Sustained blockade of neurokinin-1 receptors enhances serotonin neurotransmission. Biol Psychiatry 50:191–199
Hammack SE, Richey KJ, Schmid MJ, LoPresti ML, Watkins LR, Maier SF (2002) The role of corticotropin-releasing hormone in the dorsal raphe nucleus in mediating the behavioral consequences of uncontrollable stress. J Neurosci 22:1020–1026
Heisler LK, Pronchuk N, Nonogaki K, Zhou L, Raber J, Tung L, Yeo GS, O’Rahilly S, Colmers WF, Elmquist JK, Tecott LH (2007) Serotonin activates the hypothalamic-pituitary-adrenal axis via serotonin 2C receptor stimulation. J Neurosci 27:6956–6964
Hochberg Y (1988) A sharper Bonferroni procedure for multiple tests of significance. Biometrika 75:800–803
Hof PR, Young WG, Bloom FE, Belichenko PV, Celio MR (2000) Comparative cytoarchitectonic atlas of the C57BL/6 and 129/Sv mouse brains. Elsevier Amsterdam
Jessop DS (1999) Review: Central non-glucocorticoid inhibitors of the hypothalamo-pituitary-adrenal axis. J Endocrinol 160:169–180
Kramer MS, Cutler N, Feighner J, Shrivastava R, Carman J, Sramek JJ et al (1998) Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science 281:1640–1645
Lacoste B, Riad M, Descarries L (2006) Immunocytochemical evidence for the existence of substance P receptor (NK1) in serotonin neurons of rat and mouse dorsal raphe nucleus. Eur J Neurosci 23:2947–2958
Larsen PJ, Jessop D, Patel H, Lightman SL, Chowdrey HS (1993) Substance P inhibits the release of anterior pituitary adrenocorticotrophin via a central mechanism involving corticotrophin-releasing factor-containing neurons in the hypothalamic paraventricular nucleus. J Neuroendocrinol 5:99–105
Lightman SL, Windle RJ, Ma XM, Harbuz MS, Shanks NM, Julian MD, Wood SA, Kershaw YM, Ingram CD (2002) Hypothalamic-pituitary-adrenal function. Arch Physiol Biochem 110:90–93
Liposits Z, Phelix C, Paull WK (1987) Synaptic interaction of serotonergic axons and corticotropin releasing factor (CRF) synthesizing neurons in the hypothalamic paraventricular nucleus of the rat. A light and electron microscopic immunocytochemical study. Histochemistry 86:541–549
Lowry CA (2002) Functional subsets of serotonergic neurones: implications for control of the hypothalamic-pituitary-adrenal axis. J Neuroendocrinol 14:911–923
Lowry R, Rosebrough N, Farr A, Randall R (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Lowry CA, Rodda JE, Lightman SL, Ingram CD (2000) Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J Neurosci 20:7728–7736
Lowry CA, Plant A, Shanks N, Ingram CD, Lightman SL (2003) Anatomical and functional evidence for a stress-responsive, monoamine-accumulating area in the dorsomedial hypothalamus of adult rat brain Horm Behav 43:254–262
Mahar I, Bambico FR, Mechawar N, Nobrega JN (2014) Stress, serotonin, and hippocampal neurogenesis in relation to depression and antidepressant effects. Neurosci Biobehav Rev 38:173–192. https://doi.org/10.1016/j.neubiorev.2013.11.009
Malagié I, Trillat AC, Jacquot C, Gardier AM (1995) Effects of acute fluoxetine on extracellular serotonin levels in the raphe: an in vivo microdialysis study. Eur J Pharmacol 286:213–217
Maubach KA, Martin K, Chicchi G, Harrison T, Wheeldon A, Swain CJ, Cumberbatch MJ, Rupniak NM, Seabrook GR (2002) Chronic substance P (NK1) receptor antagonist and conventional antidepressant treatment increases burst firing of monoamine neurones in the locus coeruleus. Neuroscience 109:609–617
Miklós IH, Kovács KJ (2002) GABAergic innervation of corticotropin-releasing hormone (CRH)-secreting parvocellular neurons and its plasticity as demonstrated by quantitative immunoelectron microscopy.Neuroscience 113:581–592
Mo B, Feng N, Renner K, Forster G (2008) Restraint stress increases serotonin release in the central nucleus of the amygdala via activation of corticotropin-releasing factor receptors. Brain Res Bull 76:493–498. https://doi.org/10.1016/j.brainresbull.2008.02.011
Morgane PJ, Galler JR, Mokler DJ (2005) A review of systems and networks of the limbic forebrain/limbic midbrain. Prog Neurobiol 75:143–160
Palkovits M (1973) Isolated removal of hypothalamic or other brain nuclei of the rat. Brain Res 59:449–450
Plotsky PM, Owens MJ, Nemeroff CB (1998) Psychoneuroendocrinology of depression. Hypothalamic-pituitary-adrenal axis. Psychiatr Clin North Am 21:293–307
Ratti E, Bellew K, Bettica P, Bryson H, Zamuner S, Archer G, Squassante L, Bye A, Trist D, Krishnan KR, Fernandes S (2011) Results from 2 randomized, double-blind, placebo-controlled studies of the novel NK1 receptor antagonist casopitant in patients with major depressive disorder. J Clin Psychopharmacol 31:727–733. https://doi.org/10.1097/JCP.0b013e31823608ca Erratum J Clin Psychopharmacol). (2012) 32:185.
Roche M, Kerr DM, Hunt SP, Kelly JP (2012) Neurokinin-1 receptor deletion modulates behavioural and neurochemical alterations in an animal model of depression. Behav Brain Res 228:91–98. https://doi.org/10.1016/j.bbr.2011.11.035
Rupniak NM (2002) Elucidating the antidepressant actions of substance P (NK1 receptor) antagonists. Curr Opin Invest Drugs 3:257–261.
Rupniak NM, Kramer MS (1999) Discovery of the antidepressant and anti-emetic efficacy of substance P receptor (NK1) antagonists. Trends Pharmacol Sci 20:485–490
Rupniak NMJ, Kramer MS (2017) NK1 receptor antagonists for depression: Why a validated concept was abandoned. J Affect Disord 223:121–125. https://doi.org/10.1016/j.jad.2017.07.042
Santarelli L, Gobbi G, Debs PC, Sibille ET, Blier P, Hen R, Heath MJ (2001) Genetic and pharmacological disruption of neurokinin 1 receptor function decreases anxiety-related behaviors and increases serotonergic function. Proc Natl Acad Sci USA 98:1912–1917
Sawchenko PE, Swanson LW, Steinbusch HW, Verhofstad AA (1983) The distribution and cells of origin of serotonergic inputs to the paraventricular and supraoptic nuclei of the rat. Brain Res 277:355–360
Smythe GA, Bradshaw JE, Vining RF (1983) Hypothalamic monoamine control of stress-induced adrenocorticotropin release in the rat. Endocrinology 113:1062–1071
Ströhle A, Holsboer F (2003) Stress responsive neurohormones in depression and anxiety. Pharmacopsychiatry 36(3):S207-S214
Swanson LW (1987) The hypothalamus. In: Björklund A, Swanson T.Hökfelt, L.W. (eds) Vol integrated systems of the CNS. Part I. Handbook of Chemical Neuroanatomy, 5. Elsevier, Amsterdam, pp 1–124
Unger T, Carolus S, Demmert G, Ganten D, Lang RE, Maser-Gluth C, Steinberg H, Veelken R (1988) Substance P induces a cardiovascular defense reaction in the rat: pharmacological characterization. Circ Res 63:812–820
Valentino RJ, Bey V, Pernar L, Commons KG (2003) Substance P Acts through local circuits within the rat dorsal raphe nucleus to alter serotonergic neuronal activity. J Neurosci 23:7155–7159
Vecsei P (1979) Glucocorticoids: cortisol, corticosterone, compound S and their metabolites. In: Jaffe BM, Behrman HR (eds) Methods of hormone radioimmunoassay 2nd Edn. Academic Press New York, 767–796.
Waselus M, Valentino RJ, Van Bockstaele EJ (2011) Collateralized dorsal raphe nucleus projections: a mechanism for the integration of diverse functions during stress. J Chem Neuroanat 41:266–280. https://doi.org/10.1016/j.jchemneu.2011.05.011
Womack MD, Barrett-Jolley R (2007) Activation of paraventricular nucleus neurones by the dorsomedial hypothalamus via a tachykinin pathway in rats. Exp Physiol 92:671–676
Womack MD, Morris R, Gent TC, Barrett-Jolley R (2007) Substance P targets sympathetic control neurons in the paraventricular nucleus. Circ Res 100:1650–1658
Acknowledgements
The authors would like to thank Ms. Britta Schwarten for her excellent technical assistance.
Author information
Authors and Affiliations
Contributions
Juraj Culman conceived and designed the study, isolated brain nuclei, supervised the study and drafted and approved the article. Stephan Mühlenhoff performed experimental work (5-HT determination and CRH expression). Annegret Blume performed experimental work (ACTH determination, CRH expression). Jürgen Hedderich performed statistical analyses. Ulf Lützen contributed to the design of experiments and approved the manuscript. Stephen P Hunt provided mice for the study and made critical revision of the manuscript. Nadia M.J. Rupniak revised the manuscript for intellectual content and contributed to creating its final version. Yi Zhao conceived the work, performed the experimental work (ACTH and indole determination) and collected most of the data.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Culman, J., Mühlenhoff, S., Blume, A. et al. The Hypothalamic–Pituitary–Adrenal Axis and Serotonin Metabolism in Individual Brain Nuclei of Mice with Genetic Disruption of the NK1 Receptor Exposed to Acute Stress. Cell Mol Neurobiol 38, 1271–1281 (2018). https://doi.org/10.1007/s10571-018-0594-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-018-0594-5