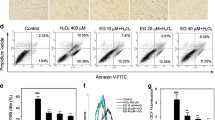
Abstract
Oxidative stress-induced neuronal apoptosis plays an important role in many neurodegenerative disorders. In this study, we have shown that indirubin-3-oxime, a derivative of indirubin originally designed for leukemia therapy, could prevent hydrogen peroxide (H2O2)-induced apoptosis in both SH-SY5Y cells and primary cerebellar granule neurons. H2O2 exposure led to the increased activities of glycogen synthase kinase 3β (GSK3β) and extracellular signal-regulated kinase (ERK) in SH-SY5Y cells. Indirubin-3-oxime treatment significantly reversed the altered activity of both the PI3-K/Akt/GSK3β cascade and the ERK pathway induced by H2O2. In addition, both GSK3β and mitogen-activated protein kinase inhibitors significantly prevented H2O2-induced neuronal apoptosis. Moreover, specific inhibitors of the phosphoinositide 3-kinase (PI3-K) abolished the neuroprotective effects of indirubin-3-oxime against H2O2-induced neuronal apoptosis. These results strongly suggest that indirubin-3-oxime prevents H2O2-induced apoptosis via concurrent inhibiting GSK3β and the ERK pathway in SH-SY5Y cells, providing support for the use of indirubin-3-oxime to treat neurodegenerative disorders caused or exacerbated by oxidative stress.










Similar content being viewed by others
Abbreviations
- ANOAVA:
-
Analysis of variance
- CGNs:
-
Cerebellar granule neurons
- ERK:
-
Extracellular signal-regulated kinase
- FBS:
-
Fetal bovine serum
- FDA:
-
Fluorescein diacetate
- GSK3β:
-
Glycogen synthase kinase 3β
- H2O2 :
-
Hydrogen peroxide
- LDH:
-
Lactate dehydrogenase
- MAPK:
-
Mitogen-activated protein kinase
- MTT:
-
3(4,5-Dimethylthiazol-2-yl)-2.5-diphenyltetrazolium bromide
- NO:
-
Nitric oxide
- PBS:
-
Phosphate-buffered saline
- PI:
-
Propidium iodide
- PI3-K:
-
Phosphoinositide 3-kinase
- ROS:
-
Reactive oxygen species
References
Bachis A, Colangelo AM, Vicini S et al (2001) Interleukin-10 prevents glutamate-mediated cerebellar granule cell death by blocking caspase-3-like activity. J Neurosci 21:3104–3112
Bhat AH, Dar KB, Anees S et al (2015) Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases: a mechanistic insight. Biomed Pharmacother 74:101–110
Cui W, Li W, Zhao Y et al (2011) Preventing H2O2-induced apoptosis in cerebellar granule neurons by regulating the VEGFR-2/Akt signaling pathway using a novel dimeric antiacetylcholinesterase bis(12)-hupyridone. Brain Res 1394:14–23
Cui W, Zhang Z, Li W et al (2012) Unexpected neuronal protection of SU5416 against 1-methyl-4-phenylpyridinium ion-induced toxicity via inhibiting neuronal nitric oxide synthase. PLoS One 7:e46253
Cui W, Zhang Z, Li W et al (2013) The anti-cancer agent SU4312 unexpectedly protects against MPP+-induced neurotoxicity via selective and direct inhibition of neuronal NOS. Br J Pharmacol 168:1201–1214
Cui W, Zhang ZJ, Hu SQ et al (2014) Sunitinib produces neuroprotective effect via inhibiting nitric oxide overproduction. CNS Neurosci Ther 20:244–252
Ding Y, Qiao A, Fan GH (2010) Indirubin-3′-monoxime rescues spatial memory deficits and attenuates β-amyloid-associated neuropathology in a mouse model of Alzheimer’s disease. Neurobiol Dis 39:156–168
Eisenbrand G, Hippe F, Jakobs S, Muehlbeyer S (2004) Molecular mechanisms of indirubin and its derivatives: novel anticancer molecules with their origin in traditional Chinese phytomedicine. J Cancer Res Clin Oncol 130:627–635
Fatokun AA, Stone TW, Smith RA (2007) Cell death in rat cerebellar granule neurons induced by hydrogen peroxide in vitro: mechanisms and protection by adenosine receptor ligands. Brain Res 1132:193–202
Frebel K, Wiese S (2006) Signalling molecules essential for neuronal survival and differentiation. Biochem Soc Trans 34:1287–1290
Gao Y, Dong C, Yin J, Shen J, Tian J, Li C (2012) Neuroprotective effect of fucoidan on H2O2-induced apoptosis in PC12 cells via activation of PI3K/Akt pathway. Cell Mol Neurobiol 32:523–529
Gonzalez-Polo RA, Soler G, Fuentes JM (2004) MPP+: mechanism for its toxicity in cerebellar granule cells. Mol Neurobiol 30:253–264
Heras-Sandoval D, Perez-Rojas JM, Hernandez-Damian J, Pedraza-Chaverri J (2014) The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell Signal 26:2694–2701
Hu SQ, Cui W, Xu DP et al (2013) Substantial neuroprotection against K+ deprivation-induced apoptosis in primary cerebellar granule neurons by novel dimer bis(propyl)-cognitin via the activation of VEGFR-2 signaling pathway. CNS Neurosci Ther 19:764–772
Hu S, Wang R, Cui W et al (2014) Inhibiting β-amyloid-associated Alzheimer’s pathogenesis in vitro and in vivo by a multifunctional dimeric bis(12)-hupyridone derived from its natural analogue. J Mol Neurosci 55:1014–1021
Hu S, Cui W, Zhang Z et al (2015) Indirubin-3-oxime effectively prevents 6OHDA-induced neurotoxicity in PC12 cells via activating MEF2D through the inhibition of GSK3β. J Mol Neurosci 57:561–570
Jiang H, Zhang L, Koubi D et al (2005) Roles of Ras-Erk in apoptosis of PC12 cells induced by trophic factor withdrawal or oxidative stress. J Mol Neurosci 25:133–140
Jones KH, Senft JA (1985) An improved method to determine cell viability by simultaneous staining with fluorescein diacetate–propidium iodide. J Histochem Cytochem 33:77–79
Jung HJ, Nam KN, Son MS et al (2010) Indirubin-3′-oxime inhibits inflammatory activation of rat brain microglia. Neurosci Lett 487:139–143
Kim GH, Kim JE, Rhie SJ, Yoon S (2015) The role of oxidative stress in neurodegenerative diseases. Exp Neurobiol 24:325–340
Lee KY, Koh SH, Noh MY, Park KW, Lee YJ, Kim SH (2007) Glycogen synthase kinase-3β activity plays very important roles in determining the fate of oxidative stress-inflicted neuronal cells. Brain Res 1129:89–99
Li X, Song L, Jope RS (1996) Cholinergic stimulation of AP-1 and NFκB transcription factors is differentially sensitive to oxidative stress in SH-SY5Y neuroblastoma: relationship to phosphoinositide hydrolysis. J Neurosci 16:5914–5922
Lin X, Wu S, Wang Q et al (2016) Saikosaponin-d Reduces HO-induced PC12 cell apoptosis by removing ROS and blocking MAPK-dependent oxidative damage. Cell Mol Neurobiol. doi:10.1007/s10571-016-0336-5
Luo J, Li W, Zhao Y et al (2010) Pathologically activated neuroprotection via uncompetitive blockade of N-methyl-d-aspartate receptors with fast off-rate by novel multifunctional dimer bis(propyl)-cognitin. J Biol Chem 285:19947–19958
Nirmaladevi D, Venkataramana M, Chandranayaka S, Ramesha A, Jameel NM, Srinivas C (2014) Neuroprotective effects of bikaverin on H2O2-induced oxidative stress mediated neuronal damage in SH-SY5Y cell line. Cell Mol Neurobiol 34:973–985
Rivest P, Renaud M, Sanderson JT (2011) Proliferative and androgenic effects of indirubin derivatives in LNCaP human prostate cancer cells at sub-apoptotic concentrations. Chem Biol Interact 189:177–185
Selenica ML, Jensen HS, Larsen AK et al (2007) Efficacy of small-molecule glycogen synthase kinase-3 inhibitors in the postnatal rat model of tau hyperphosphorylation. Br J Pharmacol 152:959–979
Sharma S, Taliyan R (2014) Neuroprotective role of indirubin-3′-monoxime, a GSKβ inhibitor in high fat diet induced cognitive impairment in mice. Biochem Biophys Res Commun 452:1009–1015
Smith PD, Mount MP, Shree R et al (2006) Calpain-regulated p35/cdk5 plays a central role in dopaminergic neuron death through modulation of the transcription factor myocyte enhancer factor 2. J Neurosci 26:440–447
Tian X, Guo LP, Hu XL et al (2015) Protective effects of Arctium lappa L. roots against hydrogen peroxide-induced cell injury and potential mechanisms in SH-SY5Y cells. Cell Mol Neurobiol 35:335–344
Valencia A, Moran J (2004) Reactive oxygen species induce different cell death mechanisms in cultured neurons. Free Radic Biol Med 36:1112–1125
Wang W, Yang Y, Ying C et al (2007) Inhibition of glycogen synthase kinase-3β protects dopaminergic neurons from MPTP toxicity. Neuropharmacology 52:1678–1684
Yang LY, Ko WC, Lin CM et al (2005) Antioxidant N-acetylcysteine blocks nerve growth factor-induced H2O2/ERK signaling in PC12 cells. Ann N Y Acad Sci 1042:325–337
Yoon SO, Yun CH, Chung AS (2002) Dose effect of oxidative stress on signal transduction in aging. Mech Ageing Dev 123:1597–1604
Zhang S, Zhang Y, Xu L et al (2009) Indirubin-3′-monoxime inhibits β-amyloid-induced neurotoxicity in neuroblastoma SH-SY5Y cells. Neurosci Lett 450:142–146
Acknowledgments
This work was supported by the Natural Science Foundation of Zhejiang Province (LY15H310007), Research Grants Council of Hong Kong (561011 & 15101014), the Applied Research Project on Nonprofit Technology of Zhejiang Province (2016C37110), the National Natural Science Foundation of China (U1503223, 81202150, 81471398), the Ningbo International Science and Technology Cooperation Project (2014D10019), Ningbo municipal innovation team of life science and health (2015C110026), Ningbo Natural Science Foundation (2015A610219), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, the State Education Ministry, and the K. C. Wong Magna Fund in Ningbo University.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Yu, J., Zheng, J., Lin, J. et al. Indirubin-3-Oxime Prevents H2O2-Induced Neuronal Apoptosis via Concurrently Inhibiting GSK3β and the ERK Pathway. Cell Mol Neurobiol 37, 655–664 (2017). https://doi.org/10.1007/s10571-016-0402-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-016-0402-z