Abstract
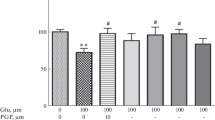
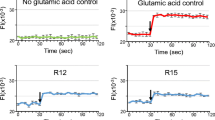
Cell-penetrating peptides (CPPs) are small peptides (typically 5–25 amino acids), which are used to facilitate the delivery of normally non-permeable cargos such as other peptides, proteins, nucleic acids, or drugs into cells. However, several recent studies have demonstrated that the TAT CPP has neuroprotective properties. Therefore, in this study, we assessed the TAT and three other CPPs (penetratin, Arg-9, Pep-1) for their neuroprotective properties in cortical neuronal cultures following exposure to glutamic acid, kainic acid, or in vitro ischemia (oxygen–glucose deprivation). Arg-9, penetratin, and TAT-D displayed consistent and high level neuroprotective activity in both the glutamic acid (IC50: 0.78, 3.4, 13.9 μM) and kainic acid (IC50: 0.81, 2.0, 6.2 μM) injury models, while Pep-1 was ineffective. The TAT-D isoform displayed similar efficacy to the TAT-L isoform in the glutamic acid model. Interestingly, Arg-9 was the only CPP that displayed efficacy when washed-out prior to glutamic acid exposure. Neuroprotection following in vitro ischemia was more variable with all peptides providing some level of neuroprotection (IC50; Arg-9: 6.0 μM, TAT-D: 7.1 μM, penetratin/Pep-1: >10 μM). The positive control peptides JNKI-1D-TAT (JNK inhibitory peptide) and/or PYC36L-TAT (AP-1 inhibitory peptide) were neuroprotective in all models. Finally, in a post-glutamic acid treatment experiment, Arg-9 was highly effective when added immediately after, and mildly effective when added 15 min post-insult, while the JNKI-1D-TAT control peptide was ineffective when added post-insult. These findings demonstrate that different CPPs have the ability to inhibit neurodamaging events/pathways associated with excitotoxic and ischemic injuries. More importantly, they highlight the need to interpret neuroprotection studies when using CPPs as delivery agents with caution. On a positive note, the cytoprotective properties of CPPs suggests they are ideal carrier molecules to deliver neuroprotective drugs to the CNS following injury and/or potential neuroprotectants in their own right.



Similar content being viewed by others
References
Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, Wang YT, Salter MW, Tymianski M (2002) Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science 298:846–850
ARAMIS (2011) Project number: 8909.1. http://www.aramis.admin.ch. Accessed 2 Nov 2013
Arthur PG, Matich GP, Pang WW, Yu DY, Bogoyevitch MA (2007) Necrotic death of neurons following an excitotoxic insult is prevented by a peptide inhibitor of c-jun N-terminal kinase. J Neurochem 102:65–76
Ashpole NM, Hudmon A (2011) Excitotoxic neuroprotection and vulnerability with CaMKII inhibition. Mol Cell Neurosci 46:720–730
Borsello T, Clarke PG, Hirt L, Vercelli A, Repici M, Schorderet DF, Bogousslavsky J, Bonny C (2003) A peptide inhibitor of c-Jun N-terminal kinase protects against excitotoxicity and cerebral ischemia. Nat Med 9:1180–1186
Cardozo AK, Buchillier V, Mathieu M, Chen J, Ortis F, Ladrière L, Allaman-Pillet N, Poirot O, Kellenberger S, Beckmann JS, Eizirik DL, Bonny C, Maurer F (2007) Cell-permeable peptides induce dose-and length-dependent cytotoxic effects. Biochim Biophys Acta 1768:2222–2234
Colombo A, Repici M, Pesaresi M, Santambrogio S, Forloni G, Borsello T (2007) The TAT-JNK inhibitor peptide interferes with beta amyloid protein stability. Cell Death Differ 14:1845–1848
Craig AJ, Meloni BP, Watt PM, Knuckey NW (2011) Attenuation of neuronal death by peptide inhibitors of AP-1 activation in acute and delayed in vitro ischaemia (oxygen/glucose deprivation) models. Int J Pept Res Ther 17:1–6
Doeppner TR, Nagel F, Dietz GPH, Weise J, Tönges L, Schwarting S, Bähr M (2009) TAT-HSP70-mediated neuroprotection and increased survival of neuronal precursor cells after focal cerebral ischemia in mice. J Cereb Blood Flow Metab 29:1187–1196
Dolgin E (2012) To serve and neuroprotect. Nat Med 18:1003–1006
Ferrer-Montiel AV, Merino JM, Blondelle SE, Perez-Payà E, Houghten RA, Montal M (1998) Selected peptides targeted to the NMDA receptor channel protect neurons from excitotoxic death. Nat Biotechnol 16:286–291
Frankel AD, Pabo CO (1988) Cellular uptake of the TAT protein from human immunodeficiency virus. Cell 55:1189–1193
Green M, Loewenstein PM (1988) Autonomous functional domains of chemically synthesized human immunodeficiency virus TAT trans-activator protein. Cell 55:1179–1188
Hirose H, Takeuchi T, Osakada H, Pujals S, Katayama S, Nakase I, Kobayashi S, Haraguchi T, Futaki S (2012) Transient focal membrane deformation induced by arginine-rich peptides leads to their direct penetration into cells. Mol Ther 20:984–993
Ho A, Schwarze SR, Mermelstein SJ, Waksman G, Dowdy SF (2001) Synthetic protein transduction domains: enhanced transduction potential in vitro and in vivo. Cancer Res 61:474–477
Kacprzak MM, Peinado JR, Than ME, Appel J, Henrich S, Lipkind G, Houghten RA, Bode W, Lindberg I (2004) Inhibition of furin by polyarginine-containing peptides: nanomolar inhibition by nona-d-arginine. J Biol Chem 279:36788–36794
Kilic Ü, Kilic E, Dietz GPH, Bähr M (2003) Intravenous TAT-GDNF is protective after focal cerebral ischemia in mice. Stroke 34:1304–1310
Lai Y, Du L, Dunsmore KE, Jenkins LW, Wong HR, Clark RS (2005) Selectively increasing inducible heat shock protein 70 via TAT-protein transduction protects neurons from nitrosative stress and excitotoxicity. J Neurochem 94:360–366
Li W, Huang Y, Reid R, Steiner J, Malpica-Llanos T, Darden TA, Shankar SK, Mahadevan A, Satishchandra P, Nath A (2008) NMDA receptor activation by HIV-Tat protein is clade dependent. J Neurosci 28:12190–12198
Liu XM, Pei DS, Guan QH, Sun YF, Wang XT, Zhang QX, Zhang GY (2006) Neuroprotection of TAT-GluR6-9c against neuronal death induced by kainate in rat hippocampus via nuclear and non-nuclear pathways. J Biol Chem 281:17432–17445
Meade AJ, Meloni BP, Mastaglia FL, Knuckey NW (2009) The application of cell penetrating peptides for the delivery of neuroprotective peptides/proteins in experimental cerebral ischemia studies. J Exp Stroke Transl Med 2:22–40
Meade AJ, Meloni BP, Mastaglia FL, Watt PM, Knuckey NW (2010a) AP-1 inhibitory peptides attenuate in vitro cortical neuronal cell death induced by kainic acid. Brain Res 1360:8–16
Meade AJ, Meloni BP, Cross J, Bakker AJ, Fear MW, Mastaglia FL, Watt PM, Knuckey NW (2010b) AP-1 inhibitory peptides are neuroprotective following acute glutamate excitotoxicity in primary cortical neuronal cultures. J Neurochem 112:258–270
Meloni BP, Majda BT, Knuckey NW (2001) Establishment of neuronal in vitro models of ischemia in 96-well microtiter strip-plates that result in acute, progressive and delayed neuronal death. Neuroscience 108:17–26
Meloni BP, Meade AJ, Kitikomolsuk D, Knuckey NW (2011) Characterisation of neuronal cell death in acute and delayed in vitro ischemia (oxygen/glucose deprivation) models. J Neurosci Methods 195:67–74
Milletti F (2012) Cell-penetrating peptides: classes, origin, and current landscape. Drug Discov Today 17:850–860
Moschos SA, Jones SW, Perry MM, Williams AE, Erjefalt JS, Turner JJ, Barnes PJ, Sproat BS, Gait MJ, Lindsay MA (2007) Lung delivery studies using siRNA conjugated to TAT(48–60) and penetratin reveal peptide induced reduction in gene expression and induction of innate immunity. Bioconjug Chem 18:1450–1459
Nagel F, Falkenburger BH, Tönges L, Kowsky S, Pöppelmeyer C, Schulz JB, Bähr M, Dietz GP (2008) TAT-HSP70 protects dopaminergic neurons in midbrain cultures and in the substantia nigra in models of Parkinson’s disease. J Neurochem 105:853–864
Nath A, Psooy K, Martin C, Knudsen B, Magnuson DS, Haughey N, Geiger JD (1996) Identification of a human immunodeficiency virus type 1 TAT epitope that is neuroexcitatory and neurotoxic. J Virol 70:1475–1480
Palm-Apergi C, Lönn P, Dowdy SF (2012) Do cell-penetrating peptides actually “penetrate” cellular membranes? Mol Ther 20:695–697
Vaslin A, Rummel C, Clarke PG (2009) Unconjugated TAT carrier peptide protects against excitotoxicity. Neurotox Res 15:123–126
Xu W, Zhou M, Baudry M (2008) Neuroprotection by cell permeable TAT-mGluR1 peptide in ischemia: synergy between carrier and cargo sequences. Neuroscientist 14:409–414
Zhang S, Taghibiglou C, Girling K, Dong Z, Lin SZ, Lee W, Shyu WC, Wang YT (2013) Critical role of increased PTEN nuclear translocation in excitotoxic and ischemic neuronal injuries. J Neurosci 33:7997–8008
Acknowledgments
This study was supported by the Neuromuscular Foundation of Western Australia and by a Grant from the National Heart Foundation (Australia). The authors would like to acknowledge Joanne Chieng for technical assistance.
Conflict of interest
Paul Watt is the Chief Executive Officer and Richard Hopkins is the Chief Operating Officer for Phylogica Ltd Pty. Nadia Milech is a Senior Scientist working for Phylogica. Bruno Meloni is a Phylogica shareholder. The other authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Meloni, B.P., Craig, A.J., Milech, N. et al. The Neuroprotective Efficacy of Cell-Penetrating Peptides TAT, Penetratin, Arg-9, and Pep-1 in Glutamic Acid, Kainic Acid, and In Vitro Ischemia Injury Models Using Primary Cortical Neuronal Cultures. Cell Mol Neurobiol 34, 173–181 (2014). https://doi.org/10.1007/s10571-013-9999-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-013-9999-3