Abstract
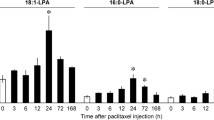
Lysophosphatidic acid (LPA) is a bioactive lipid mediator that exerts a wide range of biological actions. In recent decades, LPA has been demonstrated as an important initiator of neuropathic pain based on the mechanisms of LPA-induced feed-forward LPA amplification. In this study, we examined the possible involvement of interleukin (IL)-1β in such LPA production. Intrathecal (i.t.) LPA injection rapidly increased the expression of IL-1β mRNA in the spinal dorsal horn as early as 0.5 h after injection, and the level reached peak at 2 h. Through a developed quantitative mass spectrometry for detecting LPA species, the elevated levels of 18:1, 16:0, and 18:0 LPA in the spinal dorsal horn were observed at 3 h after 18:1 LPA injection and this elevation was completely blocked by the pretreatment of IL-1β-neutralizing antibody. Moreover, enzyme assay experiments showed that LPA (i.t.) significantly activated calcium-independent phospholipase A2 (iPLA2) and cytosolic phospholipase A2 (cPLA2) in the spinal dorsal horn at 1 and 2 h, respectively, and these biochemical changes were also significantly inhibited by IL-1β-neutralizing antibody. Similarly, IL-1β-neutralizing antibody reversed LPA-induced neuropathic pain-like behavior. These findings suggest that the early release of IL-1β is involved in LPA-induced amplification of LPA production, which underlies the initial mechanisms of LPA-induced neuropathic pain.




Similar content being viewed by others
Abbreviations
- LPA:
-
Lysophosphatidic acid
- DRG:
-
Dorsal root ganglion
- i.t.:
-
Intrathecal
- LPC:
-
Lysophosphatidylcholine
- cPLA2 :
-
Cytosolic phospholipase A2
- iPLA2 :
-
Calcium-independent phospholipase A2
- IL-1β:
-
Interleukin-1β
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- MALDI-TOFMS:
-
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
- PLA1 :
-
Phospholipase A1
References
Ahn DK, Lee SY, Han SR, Ju JS, Yang GY, Lee MK, Youn DH, Bae YC (2009) Intratrigeminal ganglionic injection of LPA causes neuropathic pain-like behavior and demyelination in rats. Pain 146:114–120
Aoki J (2004) Mechanisms of lysophosphatidic acid production. Semin Cell Dev Biol 15:477–489
Aoki J, Taira A, Takanezawa Y et al (2002) Serum lysophosphatidic acid is produced through diverse phospholipase pathways. J Biol Chem 277:48737–48744
Aoki J, Inoue A, Okudaira S (2008) Two pathways for lysophosphatidic acid production. Biochim Biophys Acta 1781:513–518
Chan LC, Peters W, Xu Y, Chun J, Farese RV Jr, Cases S (2007) LPA3 receptor mediates chemotaxis of immature murine dendritic cells to unsaturated lysophosphatidic acid (LPA). J Leukoc Biol 82:1193–1200
Choi JW, Chun J (2013) Lysophospholipids and their receptors in the central nervous system. Biochim Biophys Acta 1831:20–32
Choi JW, Herr DR, Noguchi K et al (2010) LPA receptors: subtypes and biological actions. Annu Rev Pharmacol Toxicol 50:157–186
Eder C (2009) Mechanisms of interleukin-1beta release. Immunobiology 214:543–553
Goldshmit Y, Munro K, Leong SY, Pebay A, Turnley AM (2010) LPA receptor expression in the central nervous system in health and following injury. Cell Tissue Res 341:23–32
Gupta GP, Massague J (2004) Platelets and metastasis revisited: a novel fatty link. J Clin Investig 114:1691–1693
Hanada M, Sugiura Y, Shinjo R, Masaki N, Imagama S, Ishiguro N, Matsuyama Y, Setou M (2012) Spatiotemporal alteration of phospholipids and prostaglandins in a rat model of spinal cord injury. Anal Bioanal Chem 403:1873–1884
Hargreaves K, Dubner R, Brown F, Flores C, Joris J (1988) A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 32:77–88
Hayashi Y, Kawaji K, Sun L, Zhang X, Koyano K, Yokoyama T, Kohsaka S, Inoue K, Nakanishi H (2011) Microglial Ca(2+)-activated K(+) channels are possible molecular targets for the analgesic effects of S-ketamine on neuropathic pain. J Neurosci 31:17370–17382
Hylden JL, Wilcox GL (1980) Intrathecal morphine in mice: a new technique. Eur J Pharmacol 67:313–316
Inoue M, Rashid MH, Fujita R, Contos JJ, Chun J, Ueda H (2004) Initiation of neuropathic pain requires lysophosphatidic acid receptor signaling. Nat Med 10:712–718
Inoue M, Ma L, Aoki J, Ueda H (2008) Simultaneous stimulation of spinal NK1 and NMDA receptors produces LPC which undergoes ATX-mediated conversion to LPA, an initiator of neuropathic pain. J Neurochem 107:1556–1565
Kashimoto R, Yamanaka H, Kobayashi K, Okubo M, Yagi H, Mimura O, Noguchi K (2013) Phosphorylation of ezrin/radixin/moesin (ERM) protein in spinal microglia following peripheral nerve injury and lysophosphatidic acid administration. Glia 61:338–348
Kawasaki Y, Xu ZZ, Wang X et al (2008) Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat Med 14:331–336
Lee HL, Lee KM, Son SJ, Hwang SH, Cho HJ (2004) Temporal expression of cytokines and their receptors mRNAs in a neuropathic pain model. NeuroReport 15:2807–2811
Lee C-W, Rivera R, Dubin AE, Chun J (2007) LPA4/GPR23 is a lysophosphatidic acid (LPA) receptor utilizing Gs-, Gq/Gi-mediated calcium signaling and G12/13-mediated rho activation. J Biol Chem 282:4310–4317
Lin M-E, Herr DR, Chun J (2010) Lysophosphatidic acid (LPA) receptors: signaling properties and disease relevance. Prostaglandins Other Lipid Mediat 91:130–138
Ma L, Matsumoto M, Xie W, Inoue M, Ueda H (2009a) Evidence for lysophosphatidic acid 1 receptor signaling in the early phase of neuropathic pain mechanisms in experiments using Ki-16425, a lysophosphatidic acid 1 receptor antagonist. J Neurochem 109:603–610
Ma L, Uchida H, Nagai J, Inoue M, Chun J, Aoki J, Ueda H (2009b) Lysophosphatidic acid-3 receptor-mediated feed-forward production of lysophosphatidic acid: an initiator of nerve injury-induced neuropathic pain. Mol Pain 5:64
Ma L, Nagai J, Ueda H (2010a) Microglial activation mediates de novo lysophosphatidic acid production in a model of neuropathic pain. J Neurochem 115:643–653
Ma L, Uchida H, Nagai J, Inoue M, Aoki J, Ueda H (2010b) Evidence for de novo synthesis of lysophosphatidic acid in the spinal cord through phospholipase A2 and autotaxin in nerve injury-induced neuropathic pain. J Pharmacol Exp Ther 333:540–546
Ma L, Nagai J, Sekino Y, Goto Y, Nakahira S, Ueda H (2012) Single application of A2 NTX, a botulinum toxin A2 subunit, prevents chronic pain over long periods in both diabetic and spinal cord injury-induced neuropathic pain models. J Pharmacol Sci 119:282–286
Ma L, Nagai J, Chun J, Ueda H (2013) An LPA species (18:1 LPA) plays key roles in the self-amplification of spinal LPA production in the peripheral neuropathic pain model. Molecular pain 9:29
Mills GB, Moolenaar WH (2003) The emerging role of lysophosphatidic acid in cancer. Nat Rev Cancer 3:582–591
Morishige J-I, Urikura M, Takagi H, Hirano K, Koike T, Tanaka T, Satouchi K (2010) A clean-up technology for the simultaneous determination of lysophosphatidic acid and sphingosine-1-phosphate by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry using a phosphate-capture molecule, Phos-tag. Rapid Commun Mass Spectrom 24:1075–1084
Noguchi K, Herr D, Mutoh T, Chun J (2009) Lysophosphatidic acid (LPA) and its receptors. Curr Opin Pharmacol 9:15–23
Okudaira S, Yukiura H, Aoki J (2010) Biological roles of lysophosphatidic acid signaling through its production by autotaxin. Biochimie 92:698–706
Perrin FE, Lacroix S, Avilés-Trigueros M, David S (2005) Involvement of monocyte chemoattractant protein-1, macrophage inflammatory protein-1alpha and interleukin-1beta in Wallerian degeneration. Brain 128:854–866
Reeve AJ, Patel S, Fox A, Walker K, Urban L (2000) Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain 4:247–257
Samad TA, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ (2001) Interleukin-1[beta]-mediated induction of Cox-2 in the CNS contributes to inflammatory pain hypersensitivity. Nature 410:471–475
Shan L, Jaffe K, Li S, Davis L (2008) Quantitative determination of lysophosphatidic acid by LC/ESI/MS/MS employing a reversed phase HPLC column. J Chromatogr B 864:22–28
Sugiura T, Nakane S, Kishimoto S, Waku K, Yoshioka Y, Tokumura A (2002) Lysophosphatidic acid, a growth factor-like lipid, in the saliva. J Lipid Res 43:2049–2055
Sun L, Wu Z, Hayashi Y, Peters C, Tsuda M, Inoue K, Nakanishi H (2012) Microglial cathepsin B contributes to the initiation of peripheral inflammation-induced chronic pain. J Neurosci 32:11330–11342
Sung CS, Wen ZH, Chang WK, Chan KH, Ho ST, Tsai SK, Chang YC, Wong CS (2005) Inhibition of p38 mitogen-activated protein kinase attenuates interleukin-1beta-induced thermal hyperalgesia and inducible nitric oxide synthase expression in the spinal cord. J Neurochem 94:742–752
Tager AM, LaCamera P, Shea BS et al (2008) The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med 14:45–54
Takeda H, Kawasaki A, Takahashi M, Yamada A, Koike T (2003) Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry of phosphorylated compounds using a novel phosphate capture molecule. Rapid Commun Mass Spectrom 17:2075–2081
Tanaka T, Tsutsui H, Hirano K, Koike T, Tokumura A, Satouchi K (2004) Quantitative analysis of lysophosphatidic acid by time-of-flight mass spectrometry using a phosphate-capture molecule. J Lipid Res 45:2145–2150
Tokumura A, Miyake M, Nishioka Y, Yamano S, Aono T, Fukuzawa K (1999) Production of Lysophosphatidic Acids by Lysophospholipase D in Human Follicular Fluids of In Vitro Fertilization Patients. Biol Reprod 61:195–199
Uceyler N, Tscharke A, Sommer C (2007) Early cytokine expression in mouse sciatic nerve after chronic constriction nerve injury depends on calpain. Brain Behav Immun 21:553–560
Ueda H (2008) Peripheral mechanisms of neuropathic pain—involvement of lysophosphatidic acid receptor-mediated demyelination. Mol Pain 4:11
Ueda H, Ueda M (2011) Lysophosphatidic acid as an initiator of neuropathic pain: biosynthesis and demyelination. Clin Lipidol 6:147–158
Ueda H, Matsunaga H, Olaposi OI, Nagai J (2013) Lysophosphatidic acid: chemical signature of neuropathic pain. Biochim Biophys Acta 1831:61–73
van Corven EJ, Groenink A, Jalink K, Eichholtz T, Moolenaar WH (1989) Lysophosphatidate-induced cell proliferation: identification and dissection of signaling pathways mediated by G proteins. Cell 59:45–54
Walters JN, Bickford JS, Beachy DE, Newsom KJ, Herlihy JD, Peck MV, Qiu X, Nick HS (2011) cPLA(2)alpha gene activation by IL-1beta is dependent on an upstream kinase pathway, enzymatic activation and downstream 15-lipoxygenase activity: a positive feedback loop. Cell Signal 23:1944–1951
Wolf G, Livshits D, Beilin B, Yirmiya R, Shavit Y (2008) Interleukin-1 signaling is required for induction and maintenance of postoperative incisional pain: genetic and pharmacological studies in mice. Brain Behav Immun 22:1072–1077
Xu YJ, Aziz OA, Bhugra P, Arneja AS, Mendis MR, Dhalla NS (2003) Potential role of lysophosphatidic acid in hypertension and atherosclerosis. Can J Cardiol 19:1525–1536
Zhao Y, Natarajan V (2013) Lysophosphatidic acid (LPA) and its receptors: role in airway inflammation and remodeling. Biochim Biophys Acta 1831:86–92
Acknowledgments
We thank Prof. Akira Tokumura (Tokushima University) for the gift of LPA species (16:0, 17:0, 18:0, 20:4). We used these compounds as standards in the MALDI-TOFMS analysis in the preliminary experiments. This study was supported by Grant-in-Aid for Scientific Research on Innovative Areas from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) (23116513 to H.U.), Grant-in-Aid for Young Scientists (B) (24790576 to J.N.), Ministry of Health, Labour and Welfare, the Tokyo Biomedical Research Foundation (2011 to H.U.), Ono Pharmaceutical Company Ltd and Platform for Drug Discovery, Informatics, and Structural Life Science from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Ryo Yano, Lin Ma, and Jun Nagai contributed equally to this study.
Rights and permissions
About this article
Cite this article
Yano, R., Ma, L., Nagai, J. et al. Interleukin-1β Plays Key Roles in LPA-Induced Amplification of LPA Production in Neuropathic Pain Model. Cell Mol Neurobiol 33, 1033–1041 (2013). https://doi.org/10.1007/s10571-013-9970-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-013-9970-3