Abstract
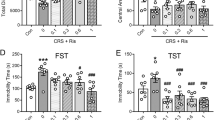
Recent evidence indicates that the administration of inhibitors of neuronal nitric oxide synthase (nNOS) induces antidepressant-like effects in animal models such as the forced swimming test (FST). However, the neural circuits involved in these effects are not yet known. Therefore, this study investigated the expression of Fos protein, a marker of neuronal activity, in the brain of rats submitted to FST and treated with the preferential nNOS inhibitor, 7-nitroindazole (7-NI), or with classical antidepressant drugs (Venlafaxine and Fluoxetine). Male Wistar rats were submitted to a forced swimming pretest (PT) and, immediately after, started receiving a sequence of three ip injections (0, 5, and 23 h after PT) of Fluoxetine (10 mg/kg), Venlafaxine (10 mg/kg), 7-NI (30 mg/kg) or respective vehicles. One hour after the last drug injection the animals were submitted to the test session, when immobility time was recorded. After the FST they were sacrificed and had their brains removed and processed for Fos immunohistochemistry. Independent group of non-stressed animals received the same drug treatments, or no treatment (naïve). 7-NI, Venlafaxine or Fluoxetine reduced immobility time in the FST, an antidepressant-like effect. None of the treatments induce significant changes in Fos expression per se. However, swimming stress induced significant increases in Fos expression in the following brain regions: medial prefrontal cortex, nucleus accumbens, locus coeruleus, raphe nuclei, striatum, hypothalamic nucleus, periaqueductal grey, amygdala, habenula, paraventricular nucleus of hypothalamus, and bed nucleus of stria terminalis. This effect was attenuated by 7-NI, Venlafaxine or Fluoxetine. These results show that 7-NI produces similar behavioral and neuronal activation effects to those of typical antidepressants, suggesting that these drugs share common neurobiological substrates.



Similar content being viewed by others
References
Aguiar DC, Guimaraes FS (2009) Blockade of NMDA receptors and nitric oxide synthesis in the dorsolateral periaqueductal gray attenuates behavioral and cellular responses of rats exposed to a live predator. J Neurosci Res 87:2418–2429
Beck CH (1995) Acute treatment with antidepressant drugs selectively increases the expression of c-Fos in the rat brain. J Psychiatry Neurosci 20:25–32
Beijamini V, Guimaraes FS (2006a) Activation of neurons containing the enzyme nitric oxide synthase following exposure to an elevated plus maze. Brain Res Bull 69:347–355
Beijamini V, Guimaraes FS (2006b) c-Fos expression increase in NADPH-diaphorase positive neurons after exposure to a live cat. Behav Brain Res 170:52–61
Bilang-Bleuel A, Rech J, De Carli S, Holsboer F, Reul JM (2002) Forced swimming evokes a biphasic response in CREB phosphorylation in extrahypothalamic limbic and neocortical brain structures in the rat. Eur J Neurosci 15:1048–1060
Bruijnzeel AW, Stam R, Compaan JC, Croiset G, Akkermans LM, Olivier B, Wiegant VM (1999) Long-term sensitization of Fos-responsivity in the rat central nervous system after a single stressful experience. Brain Res 819:15–22
Castren E (2005) Is mood chemistry? Nat Rev Neurosci 6:241–246
Charney DS (1998) Monoamine dysfunction and the pathophysiology and treatment of depression. J Clin Psychiatry 59(Suppl 14):11–14
Cryan JF, Markou A, Lucki I (2002) Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci 23:238–245
da Silva GD, Matteussi AS, dos Santos AR, Calixto JB, Rodrigues AL (2000) Evidence for dual effects of nitric oxide in the forced swimming test and in the tail suspension test in mice. Neuroreport 11(17):3699–3702
de Oliveira RM, Aparecida Del Bel E, Mamede-Rosa ML, Padovan CM, Deakin JF, Guimaraes FS (2000a) Expression of neuronal nitric oxide synthase mRNA in stress-related brain areas after restraint in rats. Neurosci Lett 289:123–126
de Oliveira RW, Del Bel EA, Guimaraes FS (2000b) Behavioral and c-Fos expression changes induced by nitric oxide donors microinjected into the dorsal periaqueductal gray. Brain Res Bull 51:457–464
Delgado PL, Moreno FA (2000) Role of norepinephrine in depression. J Clin Psychiatry 61(Suppl 1):5–12
Dhir A, Kulkarni SK (2011) Nitric oxide and major depression. Nitric Oxide 24:125–131
Duncan GE, Johnson KB, Breese GR (1993) Topographic patterns of brain activity in response to swim stress: assessment by 2-deoxyglucose uptake and expression of Fos-like immunoreactivity. J Neurosci 13:3932–3943
Duncan GE, Knapp DJ, Johnson KB, Breese GR (1996) Functional classification of antidepressants based on antagonism of swim stress-induced Fos-like immunoreactivity. J Pharmacol Exp Ther 277:1076–1089
Fossier P, Blanchard B, Ducrocq C, Leprince C, Tauc L, Baux G (1999) Nitric oxide transforms serotonin into an inactive form and this affects neuromodulation. Neuroscience 93:597–603
Gigliucci V, Buckley KN, Nunan J, O’Shea K, Harkin A (2010) A role for serotonin in the antidepressant activity of NG-Nitro-l-arginine, in the rat forced swimming test. Pharmacol Biochem Behav 94:524–533
Harkin A, Connor TJ, Walsh M, St John N, Kelly JP (2003) Serotonergic mediation of the antidepressant-like effects of nitric oxide synthase inhibitors. Neuropharmacology 44:616–623
Harkin A, Connor TJ, Burns MP, Kelly JP (2004) Nitric oxide synthase inhibitors augment the effects of serotonin re-uptake inhibitors in the forced swimming test. Eur Neuropsychopharmacol 14:274–281
Harvey BH, Retief R, Korff A, Wegener G (2006) Increased hippocampal nitric oxide synthase activity and stress responsiveness after imipramine discontinuation: role of 5HT 2A/C-receptors. Metab Brain Dis 21(2–3):211–220
Harvey BH, Duvenhage I, Viljoen F, Scheepers N, Malan SF, Wegener G, Brink CB, Petzer JP (2010) Role of monoamine oxidase, nitric oxide synthase and regional brain monoamines in the antidepressant-like effects of methylene blue and selected structural analogues. Biochem Pharmacol 80(10):1580–1591
Heiberg IL, Wegener G, Rosenberg R (2002) Reduction of cGMP and nitric oxide has antidepressant-like effects in the forced swimming test in rats. Behav Brain Res 134:479–484
Heninger GR, Delgado PL, Charney DS (1996) The revised monoamine theory of depression: a modulatory role for monoamines, based on new findings from monoamine depletion experiments in humans. Pharmacopsychiatry 29:2–11
Hindmarch I (2002) Beyond the monoamine hypothesis: mechanisms, molecules and methods. Eur Psychiatry 17(Suppl 3):294–299
Hoffman GE, Lyo D (2002) Anatomical markers of activity in neuroendocrine systems: are we all ‘Fos-ed out’? J Neuroendocrinol 14:259–268
Huang YH, Cheng CY, Hong CJ, Tsai SJ (2004) Expression of c-Fos-like immunoreactivity in the brain of mice with learned helplessness. Neurosci Lett 363:280–283
Inan SY, Yalcin I, Aksu F (2004) Dual effects of nitric oxide in the mouse forced swimming test: possible contribution of nitric oxide-mediated serotonin release and potassium channel modulation. Pharmacol Biochem Behav 77(3):457–464
Ishizuka Y, Ishida Y, Jin Q, Kato K, Kunitake T, Mitsuyama Y, Kannan H (2000) Differential profiles of nitric oxide and norepinephrine releases in the paraventricular nucleus region in response to mild footshock in rats. Brain Res 862:17–25
Jefferys D, Funder J (1996) Nitric oxide modulates retention of immobility in the forced swimming test in rats. Eur J Pharmacol 295:131–135
Joca SR, Guimaraes FS (2006) Inhibition of neuronal nitric oxide synthase in the rat hippocampus induces antidepressant-like effects. Psychopharmacology (Berl) 185:298–305
Joca SR, Padovan CM, Guimarães FS (2003) Activation of post-synaptic 5-HT(1A) receptors in the dorsal hippocampus prevents learned helplessness development. Brain Res 978(1–2):177–184
Joca SR, Zanelati T, Guimarães FS (2006) Post-stress facilitation of serotonergic, but not noradrenergic, neurotransmission in the dorsal hippocampus prevents learned helplessness development in rats. Brain Res 1087(1):67–74
Joca SR, Ferreira FR, Guimarães FS (2007) Modulation of stress consequences by hippocampal monoaminergic, glutamatergic and nitrergic neurotransmitter systems. Stress 10(3):227–249
Kovacs KJ (1998) c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int 33:287–297
Krass M, Wegener G, Vasar E, Volke V (2011) The antidepressant action of imipramine and venlafaxine involves suppression of nitric oxide synthesis. Behav Brain Res 218:57–63
Kuhn DM, Arthur RE Jr (1996) Inactivation of brain tryptophan hydroxylase by nitric oxide. J Neurochem 67:1072–1077
Kumar A, Garg R, Gaur V, Kumar P (2010) Venlafaxine involves nitric oxide modulatory mechanism in experimental model of chronic behavior despair in mice. Brain Res 1311:73–80
Li YF, Zhang YZ, Liu YQ, Wang HL, Cao JB, Guan TT, Luo ZP (2006) Inhibition of N-methyl-D-aspartate receptor function appears to be one of the common actions for antidepressants. J Psychopharmacol 20(5):629–635
Lino-de-Oliveira C, Sales AJ, Del Bel EA, Silveira MC, Guimaraes FS (2001) Effects of acute and chronic fluoxetine treatments on restraint stress-induced Fos expression. Brain Res Bull 55:747–754
Liu X, Tang X, Sanford LD (2009) Stressor controllability and Fos expression in stress regulatory regions in mice. Physiol Behav 97:321–326
Maeng S, Zarate CA Jr (2007) The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr Psychiatry Rep 9:467–474
Miyata S, Hamamura T, Lee Y, Miki M, Habara T, Oka T, Endo S, Taoka H, Kuroda S (2005) Contrasting Fos expression induced by acute reboxetine and fluoxetine in the rat forebrain: neuroanatomical substrates for the antidepressant effect. Psychopharmacology (Berl) 177:289–295
Muigg P, Hoelzl U, Palfrader K, Neumann I, Wigger A, Landgraf R, Singewald N (2007) Altered brain activation pattern associated with drug-induced attenuation of enhanced depression-like behavior in rats bred for high anxiety. Biol Psychiatry 61:782–796
Musazzi L, Racagni G, Popoli M (2011) Stress, glucocorticoids and glutamate release: Effects of antidepressant drugs. Neurochem Int 59(2):138–149
Naylor GJ, Smith AH, Connelly P (1987) A controlled trial of methylene blue in severe depressive illness. Biol Psychiatry 22:657–659
Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM (2002) Neurobiology of depression. Neuron 34:13–25
Okere CO, Waterhouse BD (2006) Acute restraint increases NADPH-diaphorase staining in distinct subregions of the rat dorsal raphe nucleus: implications for raphe serotonergic and nitrergic transmission. Brain Res 1119(1):174–181
Ons S, Marti O, Armario A (2004) Stress-induced activation of the immediate early gene Arc (activity-regulated cytoskeleton-associated protein) is restricted to telencephalic areas in the rat brain: relationship to c-Fos mRNA. J Neurochem 89:1111–1118
Pacak K, Palkovits M (2001) Stressor specificity of central neuroendocrine responses: implications for stress-related disorders. Endocr Rev 22:502–548
Paxinos G, Watson C (1997) The rat brain in stereotaxic coordinates, 3rd edn. Academic Press, New York
Pittenger C, Duman RS (2008) Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology 33:88–109
Porsolt RD, Le Pichon M, Jalfre M (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266:730–732
Prast H, Philippu A (2001) Nitric oxide as modulator of neuronal function. Prog Neurobiol 64:51–68
Price JL, Drevets WC (2010) Neurocircuitry of mood disorders. Neuropsychopharmacology 35:192–216
Racagni G, Popoli M (2008) Cellular and molecular mechanisms in the long-term action of antidepressants. Dialogues Clin Neurosci 10:385–400
Reneric JP, Lucki I (1998) Antidepressant behavioral effects by dual inhibition of monoamine reuptake in the rat forced swimming test. Psychopharmacology (Berl) 136:190–197
Roche M, Harkin A, Kelly JP (2007) Chronic fluoxetine treatment attenuates stressor-induced changes in temperature, heart rate, and neuronal activation in the olfactory bulbectomized rat. Neuropsychopharmacology 32:1312–1320
Salchner P, Singewald N (2002) Neuroanatomical substrates involved in the anxiogenic-like effect of acute fluoxetine treatment. Neuropharmacology 43:1238–1248
Salchner P, Lubec G, Engelmann M, Orlando GF, Wolf G, Sartori SB, Hoeger H, Singewald N (2004) Genetic functional inactivation of neuronal nitric oxide synthase affects stress-related Fos expression in specific brain regions. Cell Mol Life Sci 61:1498–1506
Slattery DA, Morrow JA, Hudson AL, Hill DR, Nutt DJ, Henry B (2005) Comparison of alterations in c-Fos and Egr-1 (zif268) expression throughout the rat brain following acute administration of different classes of antidepressant compounds. Neuropsychopharmacology 30:1278–1287
Steinert JR, Chernova T, Forsythe ID (2010) Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist 16:435–452
Suzuki E, Yagi G, Nakaki T, Kanba S, Asai M (2001) Elevated plasma nitrate levels in depressive states. J Affect Disord 63:221–224
Takase LF, Nogueira MI, Bland ST, Baratta M, Watkins LR, Maier SF, Fornal CA, Jacobs BL (2005) Effect of number of tailshocks on learned helplessness and activation of serotonergic and noradrenergic neurons in the rat. Behav Brain Res 162:299–306
Tokita K, Yamaji T, Hashimoto K (2011) Roles of glutamate signaling in preclinical and/or mechanistic models of depression. Pharmacol Biochem Behav
Torres G, Horowitz JM, Laflamme N, Rivest S (1998) Fluoxetine induces the transcription of genes encoding c-Fos, corticotropin-releasing factor and its type 1 receptor in rat brain. Neuroscience 87:463–477
Veening JG, Coolen LM, Spooren WJ, Joosten H, van Oorschot R, Mos J, Ronken E, Olivier B (1998) Patterns of c-Fos expression induced by fluvoxamine are different after acute vs. chronic oral administration. Eur Neuropsychopharmacol 8:213–226
Vincent SR, Kimura H (1992) Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience 46:755–784
Wegener G, Volke V, Harvey BH, Rosenberg R (2003) Local, but not systemic, administration of serotonergic antidepressants decreases hippocampal nitric oxide synthase activity. Brain Res 959:128–134
Wegener G, Harvey BH, Bonefeld B, Muller HK, Volke V, Overstreet DH, Elfving B (2010) Increased stress-evoked nitric oxide signalling in the Flinders sensitive line (FSL) rat: a genetic animal model of depression. Int J Neuropsychopharmacol 13:461–473
Wulsin AC, Herman JP, Solomon MB (2010) Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology 35:1100–1112
Yildiz F, Erden BF, Ulak G, Utkan T, Gacar N (2000) Antidepressant-like effect of 7-nitroindazole in the forced swimming test in rats. Psychopharmacology (Berl) 149:41–44
Zhou QG, Hu Y, Hua Y, Hu M, Luo CX, Han X, Zhu XJ, Wang B, Xu JS, Zhu DY (2007) Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J Neurochem 103:1843–1854
Acknowledgments
We are thankful to Eleni T. Gomes, Flavia Salata, and J. C. Aguiar for their excellent technical support, and to FAPESP (2009/18372-6; 2007/03685-3) and CNPq for the financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
Michelle Silva and Daniele C. Aguiar equally contributed to this work.
Rights and permissions
About this article
Cite this article
Silva, M., Aguiar, D.C., Diniz, C.R.A. et al. Neuronal NOS Inhibitor and Conventional Antidepressant Drugs Attenuate Stress-induced Fos Expression in Overlapping Brain Regions. Cell Mol Neurobiol 32, 443–453 (2012). https://doi.org/10.1007/s10571-011-9775-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-011-9775-1