Abstract
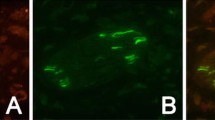
The effect of nerve growth factor (NGF) on tunicamycin (Tm)-treated neurons in the trigeminal ganglion was investigated by use of caspase-3 immunohistochemistry. In intact embryos at embryonic day 16.5, only a few caspase-3-immunoreactivity were detected in the ganglion neurons. Mean ± SE of the density of the immunoreactivity was 0.22 ± 0.03%. In contrast, the number of the immunoreactive neurons was increased at 24 h after injection of 0.5 μg Tm in 1 μl of 0.05 N NaOH solution into mouse embryos at embryonic day 15.5. The density of immunoreactivity was also increased (mean ± SE = 1.44 ± 0.11%) compared to intact and 0.05 N NaOH-treated embryos (mean ± SE = 0.35 ± 0.03%). The Tm treatment caused increase of the number of trigeminal neurons representing apoptotic profiles (intact, mean ± SE = 79.3 ± 8.5; 0.05 N NaOH, mean ± SE = 132 ± 11.5; 0.5 μg Tm, mean ± SE = 370.2 ± 64.8). In addition, NGF significantly prevented the increase of density of the immunoreactivity (mean ± SE = 0.54 ± 0.16%) and the number of apoptotic cells (mean ± SE = 146.2 ± 11.3). Saline application (without NGF) had no effect on Tm-induced increase of the immunoreactivity (mean ± SE = 1.78 ± 0.23%) or the apoptotic profiles (mean ± SE = 431.9 ± 80.5). These results indicate that Tm-induced cell death in the trigeminal ganglion is suppressed by NGF in the mouse embryo.






Similar content being viewed by others
References
Baribault TJ, Neet KE (1985) Effects of tunicamycin on NGF binding and neurite outgrowth in PC12 cells. J Neurosci Res 14:49–60
Bothwell M (1995) Functional interactions of neurotrophins and neurotrophin receptors. Annu Rev Neurosci 18:223–253
Bourke CA, Carrigan MJ (1993) Experimental tunicamycin toxicity in cattle, sheep and pigs. Aust Vet J 70:188–189
Buchman VL, Davies AM (1993) Different neurotrophins are expressed and act in a developmental sequence to promote the survival of embryonic sensory neurons. Development 118:989–1001
Chang JY, Korolev VV (1996) Specific toxicity of tunicamycin in induction of programmed cell death of sympathetic neurons. Exp Neurol 137:201–211
Duksin D, Seiberg M, Mahoney WC (1982) Inhibition of protein glycosylation and selective cytotoxicity toward virally transformed fibroblasts caused by B3-tunicamycin. Eur J Biochem 129:77–80
Elbein AD (1987) Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem 56:497–534
Finnie JW, O’shea JD (1988) Pathological and pathogenetic changes in the central nervous system of guinea pigs given tunicamycin. Acta Neuropathol 75:411–421
Fundin BT, Silos-Santiago I, Ernfors P, Fagan AM, Aldskogius H, DeChiara TM, Phillips HS, Barbacid M, Yancopoulos GD, Rice FL (1997) Differential dependency of cutaneous mechanoreceptors on neurotrophins, trk receptors, and P75 LNGFR. Dev Biol 190:94–116
Grob PM, Berlot CH, Bothwell MA (1983) Affinity labeling and partial purification of nerve growth factor receptors from rat pheochromocytoma and human melanoma cells. Proc Natl Acad Sci USA 80:6819–6823
Ichikawa H, Matsuo S, Silos-Santiago I, Sugimoto T (2000) Developmental dependency of Meissner corpuscles on trkB but not trkA or trkC. Neuroreport 11:259–262
Ichikawa H, Terayama R, Yamaai T, De Repentigny Y, Kothary R, Sugimoto T (2008) The number of nociceptors in the trigeminal ganglion but not proprioceptors in the mesencephalic trigeminal tract nucleus is reduced in dystonin deficient dystonia musculorum mice. Brain Res 1226:33–38
Jin HW, Ichikawa H, Nomura K, Mukae K, Terayama R, Yamaai T, Sugimoto T (2005) Activation of the caspase cascade underlies the rat trigeminal primary neuronal apoptosis induced by neonatal capsaicin administration. Arch Histol Cytol 68:301–310
Klein R, Smeyne RJ, Wurst W, Long LK, Auerbach BA, Joyner AL, Barbacid M (1993) Targeted disruption of the trkB neurotrophin receptor gene results in nervous system lesions and neonatal death. Cell 75:113–122
Klein R, Silos-Santiago I, Smeyne RJ, Lira SA, Brambilla R, Bryant S, Zhang L, Snider WD, Barbacid M (1994) Disruption of the neurotrophin-3 receptor gene trkC eliminates la muscle afferents and results in abnormal movements. Nature 368:249–251
Kögel D, Schomburg R, Schürmann T, Reimertz C, König HG, Poppe M, Eckert A, Müller WE, Prehn JH (2003) The amyloid precursor protein protects PC12 cells against endoplasmic reticulum stress-induced apoptosis. J Neurochem 87:248–256
Kohsaka S, Mita K, Matsuyama M, Mizuno M, Tsukuda Y (1985) Impaired development of rat cerebellum induced by neonatal injection of the glycoprotein synthesis inhibitor, tunicamycin. J Neurochem 44:406–410
Kosuge Y, Koen Y, Ishige K, Minami K, Urasawa H, Saito H, Ito Y (2003) S-allyl-L-cysteine selectively protects cultured rat hippocampal neurons from amyloid beta-protein- and tunicamycin-induced neuronal death. Neuroscience 122:885–895
Leaver DD, Schneider KM, Rand MJ, Anderson RM, Gage PW, Malbon R (1988) The neurotoxicity of tunicamycin. Toxicology 49:179–187
Lewin GR, Barde YA (1996) Physiology of the neurotrophins. Annu Rev Neurosci 19:289–317
Lindsay RM, Wiegand SJ, Altar CA, DiStefano PS (1994) Neurotrophic factors: from molecule to man. Trends Neurosci 17:182–190
Martin ZD, Oskam R, Mitra G, Copeland T, Barbacid M (1989) Molecular and biochemical characterization of the human trk proto-oncogene. Mol Cell Biol 9:24–33
Morin MJ, Bernacki RJ (1983) Biochemical effects and therapeutic potential of tunicamycin in murine L1210 leukemia. Cancer Res 43:1669–1674
Reimertz C, Kögel D, Rami A, Chittenden T, Prehn JH (2003) Gene expression during ER stress-induced apoptosis in neurons: induction of the BH3-only protein Bbc3/PUMA and activation of the mitochondrial apoptosis pathway. J Cell Biol 162:587–597
Salton SR, Shelanski ML, Greene LA (1983) Biochemical properties of the nerve growth factor-inducible large external (NILE) glycoprotein. J Neurosci 3:2420–2430
Segal RA, Greenberg ME (1996) Intracellular signaling pathways activated by neurotrophic factors. Annu Rev Neurosci 19:463–489
Shimoke K, Amano H, Kishi S, Uchida H, Kudo M, Ikeuchi T (2004a) Nerve growth factor attenuates endoplasmic reticulum stress-mediated apoptosis via suppression of caspase-12 activity. J Biochem 135:439–446
Shimoke K, Utsumi T, Kishi S, Nishimura M, Sasaya H, Kudo M, Ikeuchi T (2004b) Prevention of endoplasmic reticulum stress-induced cell death by brain-derived neurotrophic factor in cultured cerebral cortical neurons. Brain Res 1028:105–111
Silos-Santiago I, Molliver DC, Ozaki S, Smeyne RJ, Fagan AM, Barbacid M, Snider WD (1995) Non-TrkA-expressing small DRG neurons are lost in TrkA deficient mice. J Neurosci 15:5929–5942
Smeyne RJ, Klein R, Schnapp A, Long LK, Bryant S, Lewin A, Lira SA, Barbacid M (1994) Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature 368:246–249
Sugimoto T, Jin H, Fijita M, Fukunaga T, Nagaoka N, Yamaai T, Ichikawa H (2004) Induction of activated caspase-3-immunoreactivity and apoptosis in the trigeminal ganglion neurons by neonatal peripheral nerve injury. Brain Res 1017:238–243
Tolkovsky AM (1987) Newly synthesized catalytic and regulatory components of adenylate cyclase are expressed in neuritis of cultured sympathetic neurons. J Neurosci 7:110–119
Vogel P, Mcwilliam AS (1987) The neurological effects of tunicamycin may reduce its potential as an anticancer agent. Med J Aust 146:53
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ichikawa, H., Zhao, BR., Kano, M. et al. Tunicamycin-Induced Cell Death in the Trigeminal Ganglion is Suppressed by Nerve Growth Factor in the Mouse Embryo. Cell Mol Neurobiol 30, 461–467 (2010). https://doi.org/10.1007/s10571-009-9471-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-009-9471-6