Abstract
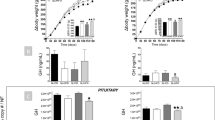
Juvenile obesity is a rising epidemic due largely to consumption of caloric dense, fat-enriched foods. Nevertheless, literature on fat-induced neuroendocrine and metabolic disturbances during adolescence, preceding obesity, is limited. This study aimed to examine early events induced by a fat diet (45% calories from saturated fat) in male rats fed the diet during the pre- and post-pubertal period. The neuroendocrine endpoints studied were the levels of circulating leptin, insulin and corticosterone, as well as their receptors in the hypothalamus and hippocampus. Hormonal levels were determined by radioimmunoassay and receptors’ levels by western blot analysis. Leptinemia was increased in pubertal rats and in adult rats fed the fat diet from weaning to adulthood, but not in those fed from puberty to adulthood. Modifications in the developmental pattern from puberty to adulthood were observed for most of the brain receptors studied. In adult animals fed the fat diet from weaning onwards, the levels of leptin receptors in the hypothalamus and glucocorticoid receptors in the hippocampus were decreased compared to chow-fed controls. Switching from fat to normal chow at puberty onset restored the diet-induced alterations on circulating leptin, but not on its hypothalamic receptors. These data suggest that when a fat-enriched diet, resembling those consumed by many teenagers, provided in rats during pubertal growth, it can longitudinally influence the actions of leptin and corticosterone in the brain. The observed alterations at a preobese state may constitute early signs of the disturbed energy balance toward overweight and obesity.


Similar content being viewed by others
References
Ahima RS, Osei SY (2001) Molecular regulation of eating behavior: new insights and prospects for therapeutic strategies. Trends Mol Med 7:205–213
Banas SM, Rouch C, Kassis N, Markaki EM, Gerozissis K (2009) A dietary fat excess alters metabolic and neuroendocrine responses before the onset of metabolic diseases. Cell Mol Neurobiol 29:157–168
Boukouvalas G, Antoniou K, Papalexi E, Kitraki E (2008) Postweaning high fat feeding affects rats’ behavior and hypothalamic pituitary adrenal axis at the onset of puberty in a sexually dimorphic manner. Neuroscience 153:373–382
Brooks SPJ, Lampi BJ (1997) Time course of enzyme changes after a switch from a high-fat to a low-fat diet. Comp Biochem Physiol Biochem Mol Biol 118:359–365
Brooks SPJ, Lampi BJ (1999) Effect of dietary fat on whole body fatty acid synthesis in weanling rats. J Nutr Biochem 10:291–298
Cha MC, Chou CJ, Boozer CN (2000) High-fat diet feeding reduces the diurnal variation of plasma leptin concentration in rats. Metabolism 49:503–507
Cruciani-Guglielmacci C, Vincent-Lamon M, Rouch C, Orosco M, Ktorza A, Magnan C (2005) Early changes in insulin secretion and action induced by high-fat diet are related to a decreased sympathetic tone. Am J Physiol Endocrinol Metab 288:E148–E154
De Kloet ER, Karst H, Joëls M (2008) Corticosteroid hormones in the central stress response: quick-and-slow. Front Neuroendocrinol 29:268–272
Dou JT, Chen M, Dufour F, Alkon DL, Zhao WQ (2005) Insulin receptor signaling in long-term memory consolidation following spatial learning. Learn Mem 12:646–655
Drake AJ, Livingstone DE, Andrew R, Seckl JR, Morton NM, Walker BR (2005) Reduced adipose glucocorticoid reactivation and increased hepatic glucocorticoid clearance as an early adaptation to high-fat feeding in Wistar rats. Endocrinology 146:913–999
Fehm HL, Kern W, Peters A (2006) The selfish brain: competition for energy resources. Prog Brain Res 153:129–140
Gerozissis K (2008) Brain insulin, energy and glucose homeostasis; genes, environment and metabolic pathologies. Eur J Pharmacol 585:38–49
Ghibaudi L, Cook J, Farley C, van Heek M, Hwa JJ (2002) Fat intake affects adiposity, comorbidity factors, and energy metabolism of sprague-dawley rats. Obes Res 10:956–963
Golden SH (2007) A review of the evidence for a neuroendocrine link between stress, depression and diabetes mellitus. Curr Diabetes Rev 3:252–259
Kaiyala KJ, Prigeon RL, Kahn SE, Woods SC, Schwartz MW (2000) Obesity induced by a high-fat diet is associated with reduced brain insulin transport in dogs. Diabetes 49:1525–1533
Kitraki E, Soulis G, Gerozissis K (2004) Impaired neuroendocrine response to stress following a short term fat-enriched diet. Neuroendocrinology 79:338–345
Kong AP, Chan NN, Chan JC (2006) The role of adipocytokines and neurohormonal dysregulation in metabolic syndrome. Curr Diabetes Rev 2:397–407
Koros C, Boukouvalas G, Gerozissis K, Kitraki E (2009) Fat diet affects leptin receptor levels in the rat cerebellum. Nutrition 25:85–87
Kraegen EW, Clark PW, Jenkins AB, Daley EA, Chisholm DJ, Storlien LH (1991) Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes 40:1397–1403
Lee Y, Wang MY, Kakuma T, Wang ZW, Babcock E, McCorkle K, Higa M, Zhou YT, Unger RH (2001) Liporegulation in diet-induced obesity. The antisteatotic role of hyperleptinemia. J Biol Chem 276:5629–5635
Levin BE, Dunn-Meynell AA (2002) Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 283:R941–R948
MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Peters JC, Hill JO (2004) Metabolic adjustments with the development, treatment, and recurrence of obesity in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 287:R288–R297
Martin RL, Perez E, He YJ, Dawson R Jr, Millard WJ (2000) Leptin resistance is associated with hypothalamic leptin receptor mRNA and protein down regulation. Metabolism 49:1479–1484
McCormick CM, Mathews IZ (2007) HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol Biochem Behav 86:220–233
Michel C, Dunn-Meynell A, Levin BE (2004) Reduced brain CRH and GR mRNA expression precedes obesity in juvenile rats bred for diet-induced obesity. Behav Brain Res 154:511–517
Münzberg H, Flier JS, Bjørbaek C (2004) Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 145:4880–4889
Niswender KD, Baskin DG, Schwartz MW (2004) Insulin and its evolving partnership with leptin in the hypothalamic control of energy homeostasis. Trends Endocrinol Metab 15:362–369
Pal R, Sahu A (2003) Leptin signaling in the hypothalamus during chronic central leptin infusion. Endocrinology 144:3789–3798
Romeo RD (2003) Puberty: a period of both organizational and activational effects of steroid hormones on neurobehavioural development. J Neuroendocrinol 15:1185–1192
Scarpace PJ, Zhang Y (2007) Elevated leptin: consequence or cause of obesity? Front Biosci 12:3531–3544
Siriani D, Mitsiou DJ, Alexis MN (2003) Overexpressed glucocorticoid receptor negatively regulates gene expression under conditions that favour accumulation of non-hormone-binding forms of the receptor. J Steroid Biochem Mol Biol 84:171–180
Sisk CL, Zehr JL (2005) Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol 26:163–174
Soulis G, Kitraki E, Gerozissis K (2005) Early neuroendocrine alterations in female rats following a diet moderately enriched in fat. Cell Mol Neurobiol 25:869–880
Spinedi E, Gaillard RC (1998) A regulatory loop between the hypothalamo-pituitary-adrenal (HPA) axis and circulating leptin: a physiological role of ACTH. Endocrinology 139:4016–4020
Tannenbaum BM, Brindley DN, Tannenbaum GS, Dallman MF, McArthur MD, Meaney MJ (1997) High-fat feeding alters both basal and stress-induced hypothalamic-pituitary-adrenal activity in the rat. Am J Physiol 273:E1168–E1177
Vegiopoulos A, Herzig S (2007) Glucocorticoids, metabolism and metabolic diseases. Mol Cell Endocrinol 275:43–61
Zhu JN, Wang JJ (2008) The cerebellum in feeding control: possible function and mechanism. Cell Mol Neurobiol 28(4):469–478
Acknowledgments
This work was supported by E.U.-European Social Funds (75%) and the Greek Ministry of Development-GSRT (25%) within the framework of the “Reinforcement Program of Human Research Manpower” (grant PENED 03ED81). IASO Hospital (Greece) is acknowledged for material supply and indirect financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Boukouvalas, G., Gerozissis, K. & Kitraki, E. Fat Feeding of Rats During Pubertal Growth Leads to Neuroendocrine Alterations in Adulthood. Cell Mol Neurobiol 30, 91–99 (2010). https://doi.org/10.1007/s10571-009-9434-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-009-9434-y