Abstract
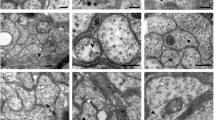
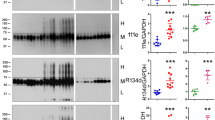
Transition of protein tau from physiologically unfolded to misfolded state represent enigmatic step in the pathogenesis of tauopathies including Alzheimer’s disease (AD). Major molecular events playing role in this process involve truncation and hyperphosphorylation of tau protein, which are accompanied by redox imbalance followed by functional deterioration of neuronal network. Recently we have developed transgenic rat model showing that expression of truncated tau causes neurofibrillary degeneration similar to that observed in brain of AD sufferers. Consequently we tested cortical and hippocampal neuronal cultures extracted from this model as a convenient tool for development of molecules able to target the mechanisms leading to and/or enhancing the process of neurodegeneration. Here we document three major pathological features typical for tauopathies and AD in cortical and hippocampal neurons from transgenic rat in vitro. First, an increased accumulation of human truncated tau in neurons; second, the hyperphosphorylation of truncated tau on the epitopes characteristic of AD (Ser202/Thr205 and Thr231); and third, increased vulnerability of the neurons to nitrative and oxidative stress. Our results show that primary neurons expressing human truncated tau could represent a cellular model for targeting tau related pathological events, namely, aberrant tau protein accumulation, tau hyperphosphorylation, and oxidative/nitrative damage. These characteristics make the model particularly suitable for detailed study of molecular mechanisms of tau induced neurodegeneration and easily applicable for drug screening.




Similar content being viewed by others
References
Aliev G, Smith MA, De la Torre JC, Perry G (2004) Mitochondria as a primary target for vascular hypoperfusion and oxidative stress in Alzheimer’s disease. Mitochondrion 4:649–663. doi:10.1016/j.mito.2004.07.018
Alonso AC, Mederlyova A, Novak N, Grundke-Iqbal I, Iqbal K (2004) Promotion of hyperphosphorylation by frontotemporal dementia tau mutations. J Biol Chem 279:34878–34881
Bertram L, Tanzi RE (2005) The genetic epidemiology of neurodegenerative disease. J Clin Invest 115:1449–1457. doi:10.1172/JCI24761
Braak H, Braak E (1991) Neuropathological staging of Alzheimer-related changes. Acta Neuropathol 82:239–259. doi:10.1007/BF00308809
Butterfield DA, Perluigi M, Sultana R (2006) Oxidative stress in Alzheimer’s disease brain: new insights from redox proteomics. Eur J Pharmacol 545:39–50. doi:10.1016/j.ejphar.2006.06.026
Cente M, Filipcik P, Pevalova M, Novak M (2006) Expression of a truncated tau protein induces oxidative stress in a rodent model of tauopathy. Eur J NeuroSci 24:1085–1090. doi:10.1111/j.1460-9568.2006.04986.x
Csokova N, Skrabana R, Liebig HD, Mederlyova A, Kontsek P, Novak M (2004) Rapid purification of truncated tau proteins: model approach to purification of functionally active fragments of disordered proteins, implication for neurodegenerative diseases. Protein Expr Purif 35:366–372. doi:10.1016/j.pep.2004.01.012
Eide L, McMurray CT (2005) Culture of adult mouse neurons. Biotechniques 38:99–104. doi:10.2144/05381RR02
Garcia-Sierra F, Ghoshal N, Quinn B, Berry RW, Binder LI (2003) Conformational changes and truncation of tau protein during tangle evolution in Alzheimer’s disease. J Alzheimers Dis 5:65–77
Götz J, Deters N, Doldissen A, Bokhari L, Ke Y, Wiesner A, Schonrock N, Ittner LM (2007) A decade of tau transgenic animal models and beyond. Brain Pathol 17:91–103. doi:10.1111/j.1750-3639.2007.00051.x
Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI (1986) Abnormal phosphorylation of the microtubule-associated protein tau in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA 83:4913–4917. doi:10.1073/pnas.83.13.4913
Horowitz PM, Patterson KR, Guillozet-Bongaarts AL, Reynolds MR, Carroll CA, Weintraub ST, Bennett DA, Cryns VL, Berry RW, Binder LI (2004) Early N-terminal changes and caspase-6 cleavage of tau in Alzheimer’s disease. J Neurosci 24:7895–7902. doi:10.1523/JNEUROSCI.1988-04.2004
Iqbal K, Alonso Adel C, Chen S, Chohan MO, El-Akkad E, Gong CX, Khatoon S, Li B, Liu F, Rahman A, Tanimukai H, Grundke-Iqbal I (2005) Tau pathology in Alzheimer disease and other tauopathies. Biochim Biophys Acta 1739:198–210
King ME (2005) Can tau filaments be both physiologically beneficial and toxic? Biochim Biophys Acta 1739:260–267
Koson P, Zilka N, Kovac A, Kovacech B, Korenova M, Filipcik P, Novak M (2008) Truncated tau expression levels determine life span of a rat model of tauopathy without causing neuronal loss or correlating with terminal neurofibrillary tangle load. Eur J Neurosci 28:239–246. doi:10.1111/j.1460-9568.2008.06329.x
Kril JJ, Patel S, Harding AJ, Halliday GM (2002) Neuron loss from the hippocampus of Alzheimer`s disease exceeds extracellular neurofibrillary tangle formation. Acta Neuropathol 103:370–376. doi:10.1007/s00401-001-0477-5
Le Corre S, Klafki HW, Plesnila N, Hübinger G, Obermeier A, Sahagún H, Monse B, Seneci P, Lewis J, Eriksen J, Zehr C, Yue M, McGowan E, Dickson DW, Hutton M, Roder HM (2006) An inhibitor of tau hyperphosphorylation prevents severe motor impairments in tau transgenic mice. Proc Natl Acad Sci USA 103:9673–9678. doi:10.1073/pnas.0602913103
Moreira PI, Smith MA, Zhu X, Honda K, Lee HG, Aliev G, Perry G (2005) Oxidative damage and Alzheimer’s disease: are antioxidant therapies useful? Drug News Perspect 18:13–19. doi:10.1358/dnp.2005.18.1.877165
Morsch R, Simon W, Coleman PD (1999) Neurons may live for decades with neurofibrillary tangles. J Neuropathol Exp Neurol 58:188–197. doi:10.1097/00005072-199902000-00008
Novak M (1994) Truncated tau protein as a new marker for Alzheimer’s disease. Acta Virol 38:173–189
Novak M, Wischik CM, Edwards P, Pannell R, Milstein C (1989) Characterization of the first monoclonal antibody against the pronase resistant core of the Alzheimer PHF. Prog Clin Biol Res 317:755–761
Novak M, Kabat J, Wischik CM (1993) Molecular characterization of the minimal protease resistant tau unit of the Alzheimer’s disease paired helical filament. EMBO J 12:365–370
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39:409–421. doi:10.1016/S0896-6273(03)00434-3
Perry G, Nunomura A, Hirai K, Zhu X, Perez M, Avila J, Castellani RJ, Atwood CS, Aliev G, Sayre LM, Takeda A, Smith MA (2002) Is oxidative damage the fundamental pathogenic mechanism of Alzheimer’s and other neurodegenerative diseases? Free Radic Biol Med 33:1475–1479. doi:10.1016/S0891-5849(02)01113-9
Schindowski K, Bretteville A, Leroy K, Bégard S, Brion JP, Hamdane M, Buée L (2006) Alzheimer’s disease-like tau neuropathology leads to memory deficits and loss of functional synapses in a novel mutated tau transgenic mouse without any motor deficits. Am J Pathol 169:599–616. doi:10.2353/ajpath.2006.060002
Selkoe DJ (2002) Alzheimer’s disease is a synaptic failure. Science 298:789–791. doi:10.1126/science.1074069
Watanabe A, Hong WK, Dohmae N, Takio K, Morishima-Kawashima M, Ihara Y (2004) Molecular aging of tau: disulfide-independent aggregation and non-enzymatic degradation in vitro and in vivo. J Neurochem 90:1302–1311. doi:10.1111/j.1471-4159.2004.02611.x
Wischik CM, Novak M, Thogersen HC, Edwards PC, Runswick MJ, Jakes R, Walker JE, Milstein C, Roth M, Klug A (1988) Isolation of a fragment of tau derived from the core of the paired helical filament of Alzheimer disease. Proc Natl Acad Sci USA 85:4506–4510. doi:10.1073/pnas.85.12.4506
Zhang YJ, Xu YF, Liu YH, Yin J, Li HL, Wang Q, Wang JZ (2006) Peroxynitrite induces Alzheimer-like tau modifications and accumulation in rat brain and its underlying mechanisms. FASEB J 20:1431–1442. doi:10.1096/fj.05-5223com
Zilka N, Filipcik P, Koson P, Vechterova L, Skrabana R, Zilkova M, Rolkova G, Kontsekova E, Novak M (2006) Truncated tau from sporadic Alzheimer’s disease suffices to drive neurofibrillary degeneration in vivo. FEBS Lett 580:3582–3588. doi:10.1016/j.febslet.2006.05.029
Acknowledgments
This work was supported by research grant: APVV No. 0634-07, LPP 0354-06, VEGA 2/7130/27, and 2/0148/08.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Filipcik, P., Cente, M., Krajciova, G. et al. Cortical and Hippocampal Neurons from Truncated Tau Transgenic Rat Express Multiple Markers of Neurodegeneration. Cell Mol Neurobiol 29, 895–900 (2009). https://doi.org/10.1007/s10571-009-9372-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-009-9372-8