Summary
1. The lateral hypothalamus (LH) and the dorsal periaqueductal gray area (dPAG) are two important brain structures involved in central cardiovascular control.
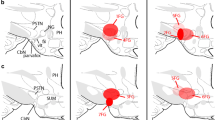
2. In the present study, we searched for possible rostrocaudal somatotopy in the neural connections from the three subdivisions of the LH (anterior—LHa; tuberal—LHt and posterior—LHp) to the different rostrocaudal portions of the dPAG.
3. The bidirectional neuronal tracer biotinylated-dextran-amine (BDA) was microinjected into different rostrocaudal coordinates of the dPAG (AP 3.4–2.7 mm) of male Wistar rats. One week later, animals were sacrificed and brain slices were processed and analyzed to detect neuronal efferent projections from the LH to the dPAG.
4. Neuronal cell body staining was observed along all the rostrocaudal axis of the LH when BDA was microinjected in more rostral dPAG coordinates. When the BDA was microinjected into more caudal dPAG regions, labeled neurons were observed only in the caudal portion of the LH.
5. Efferent projections from the LHa were directed only to the rostral portion of the dPAG. Projections from the rostral and medial portions of the LHt were also directed to the rostral dPAG, whereas both rostral and caudal dPAG received projections from the caudal portion of the LHt. Efferent projections from the anterior portion of the LHp were directed to both rostral and caudal dPAG, whereas projections from the caudal LHp were only directed to the rostral portion of the dPAG.
6. The results suggest a somatotopic correlation in LH projections to the dPAG with main connections to the rostral dPAG, which are efferent from the three divisions of the LH. More caudal regions of the dPAG received afferents only from posterior sites in the LH.
7. Moreover, the results point out to extensive and complex neural somatotopic projections from all LH subdivisions to different rostrocaudal portions of the dPAG, reinforcing the idea of significant functional interactions between the brain structures.



Similar content being viewed by others
REFERENCES
Allen, G. V., and Cechetto, D. F. (1992). Functional and anatomical organization of cardiovascular pressor and depressor sites in the lateral hypothalamic area: I. Descending projections. J. Comp. Neurol. 315:313–332.
Bandler, R., Carrive, P., and Depaulis, A. (1991). Introduction: emerging principles of organization of the midbrain periaqueductal gray matter. In Depaulis, A., and Bandler, R. (eds.), The Midbrain Periaqueductal Gray Matter: Functional, Anatomical, and Neurochemical Organization, Plenum Press, New York, pp. 1–8.
Bandler, R., and Tork, I. (1987). Midbrain periaqueductal grey region in the cat has afferent and efferent connections with solitary tract nuclei. Neurosci. Lett. 74:1–6.
Beitz, A. J. (1995). Periaqueductal gray. In Paxinos, G. (ed.), The Rat Nervous System. Academic Press, Australia, pp. 173–182.
Berk, M. L., and Finkelstein, J. A. (1983). Long descending projections of the hypothalamus in the pigeon, Columba livia. J. Comp. Neurol. 220:127–136.
Holstege, G. (1987). Some anatomical observations on the projections from the hypothalamus to brainstem and spinal cord: An HRP and autoradiographic tracing study in the cat. J. Comp. Neurol. 260:98–126.
Hosoya, Y., and Matsushita, M. (1981). Brainstem projections from the lateral hypothalamic area in the rat, as studied with autoradiography. Neurosci. Lett. 24:111–116.
Huang, Z. G., Subramanian, S. H., Balnave, R. J., Turman, A. B., and Moi Chow, C. (2000). Roles of periaqueductal gray and nucleus tractus solitarius in cardiorespiratory function in the rat brainstem. Respir. Physiol. 120:185–195.
Iwata, J., LeDoux, J. E., and Reis, D. J. (1986). Destruction of intrinsic neurons in the lateral hypothalamus disrupts the classical conditioning of autonomic but not behavioral emotional responses in the rat. Brain Res. 368:161–166.
Jenck, F., Broekkamp, C. L., and Van Delft, A. M. (1989). Opposite control mediated by central 5-HT1A and non-5-HT1A (5-HT1B or 5-HT1C) receptors on periaqueductal gray aversion. Eur. J. Pharmacol.. 161:219–221.
LeDoux, J. E., Iwata, J., Cicchetti, P., and Reis, D. J. (1988). Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 8:2517–2529.
Liebman, J. M., Mayer, D. J., and Liebeskind, J. C. (1970). Mesencephalic central gray lesions and fear-motivated behavior in rats. Brain Res. 23:353–370.
Lyon, M. (1964). The role of central midbrain structures in conditioned responding to aversive noise in the rat. J. Comp. Neurol. 122:407–429.
Mantyh, P. W. (1982). Forebrain projections to the periaqueductal gray in the monkey, with observations in the cat and rat. J. Comp. Neurol. 206:146–158.
Nashold, B. S., Jr., Wilson, W. P., and Slaughter, D. G. (1969). Sensations evoked by stimulation in the midbrain of man. J. Neurosurg. 30:14–24.
Pajolla, G. P., and de Aguiar Correa, F. M. (2004). Cardiovascular responses to the injection of L-glutamate in the lateral hypothalamus of unanesthetized or anesthetized rats. Auton. Neurosci 116:19–29.
Pajolla, G. P., Tavares, R. F., Pelosi, G. G., and Correa, F. M. (2005). Involvement of the periaqueductal gray in the hypotensive response evoked by l-glutamate microinjection in the lateral hypothalamus of unanesthetized rats. Auton. Neurosci. 122:84–93.
Paredes, J., Winters, R. W., Schneiderman, N., and McCabe, P. M. (2000). Afferents to the central nucleus of the amygdala and functional subdivisions of the periaqueductal gray: Neuroanatomical substrates for affective behavior. Brain Res. 887:157–173.
Paxinos, G., and Watson, C., (1997). The Rat Brain in Steretaxic Coordinates, Academic Press, Sydney.
Pelosi, G. G., and Corrêa, F. M. A. (2005). Cardiovascular effects of noradrenaline microinjected into the dorsal periaqueductal gray area of unanaesthetized rats. Eur. J. Neurosci. 22:3188–3194.
Saper, C. B., Swanson, L. W., and Cowan, W. M. (1979). An autoradiographic study of the efferent connections of the lateral hypothalamic area in the rat. J. Comp. Neurol. 183:689–706.
Semenenko, F. M., and Lumb, B. M. (1992). Projections of anterior hypothalamic neurones to the dorsal and ventral periaqueductal grey in the rat. Brain Res. 582:237–245.
ter Horst, G. J., Luiten, P. G., and Kuipers, F. (1984). Descending pathways from hypothalamus to dorsal motor vagus and ambiguus nuclei in the rat. J. Auton. Nerv. Syst. 11:59–75.
van der Plas, J., Wiersinga-Post, J. E., Maes, F. W., and Bohus, B. (1995). Cardiovascular effects and changes in midbrain periaqueductal gray neuronal activity induced by electrical stimulation of the hypothalamus in the rat. Brain Res. Bull. 37:645–656.
Vercelli, A., Repici, M., Garbossa, D., and Grimaldi, A. (2000). Recent techniques for tracing pathways in the central nervous system of developing and adult mammals. Brain Res. Bull. 51:11–28.
Yardley, C. P., and Hilton, S. M. (1986). The hypothalamic and brainstem areas from which the cardiovascular and behavioural components of the defence reaction are elicited in the rat. J. Auton. Nerv. Syst. 15:227–244.
ACKNOWLEDGMENTS
The authors would like to thank Ms Ivanilda A.C. Fortunato, Simone S. Guilhaume and Idalia I.B. Aguiar for technical support. Gislaine G. Pelosi is a Ph.D. student (Fapesp-02/14147-9) enrolled in the Graduation Program on Pharmacology of the School of Medicine of Ribeirão Preto and Rodrigo F. Tavares is a post-doc fellow (Fapesp 04/01270-2). This study was supported by grant from CAPES and CNPq (306381/2003-6 and 505394/2003-0).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pelosi, G.G., Tavares, R.F. & Corrêa, F.M.A. Rostrocaudal Somatotopy in the Neural Connections Between the Lateral Hypothalamus and the Dorsal Periaqueductal Gray of the Rat Brain. Cell Mol Neurobiol 26, 633–641 (2006). https://doi.org/10.1007/s10571-006-9015-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-006-9015-2