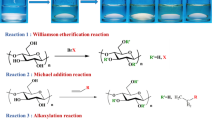
Abstract
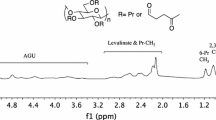
Chemical modification of cellulose is challenging due to its low reactivity and poor solubility. Halogenation followed by displacement reactions has been demonstrated to be a valuable strategy for appending new functionalities to the anhydroglucose rings of cellulose and cellulose derivatives. In this paper, we report a simple and efficient pathway to modify the inexpensive, commercial cellulose ether, hydroxyethyl cellulose (HEC). First, methanesulfonyl chloride (MsCl) in N, N-dimethyl formamide (DMF) can selectively chlorinate the terminal primary hydroxyl groups from hydroxyethyl cellulose (HEC), thereby affording high terminal chloride content. Then, the resulting chlorinated HEC undergoes displacement reactions with various nucleophiles including azide (NaN3), amine (1-methylimidazole), and thiols (3-mercaptopropionic acid and 2-mercaptoethanol). All products were characterized by NMR and FT-IR spectroscopic methods. Exploiting this strategy, we prepared a library of HEC derivatives, including cationic and anionic derivatives, which are of great interest in various applications including as surfactants, in gas separation membranes, and as crystallization inhibitors in amorphous solid dispersions for oral drug bioavailability enhancement.








Similar content being viewed by others
Data availability
Data not already included in the manuscript and supplementary material is available from the authors by request.
References
Abbas K, Amin M, Hussain MA et al (2017) Design, characterization and pharmaceutical/pharmacological applications of ibuprofen conjugates based on hydroxyethylcellulose. RSC Adv 7:50672–50679. https://doi.org/10.1039/c7ra08502h
Afratis N, Gialeli C, Nikitovic D et al (2012) Glycosaminoglycans: key players in cancer cell biology and treatment. FEBS J 279:1177–1197. https://doi.org/10.1111/j.1742-4658.2012.08529.x
Aoki N, Furuhata K-I, Saegusa Y et al (1996) Reaction of 6-bromo-6-deoxycellulose with thiols in lithium bromide-N,N-dimethylacetamide. J Appl Polym Sci 61:1173–1185. https://doi.org/10.1002/(SICI)1097-4628(19960815)61:7%3c1173::AID-APP13%3e3.0.CO;2-Z
Aoki N, Fukushima K, Kurakata H et al (1999) 6-Deoxy-6-mercaptocellulose and its S-substituted derivatives as sorbents for metal ions. React Funct Polym 42:223–233. https://doi.org/10.1016/S1381-5148(98)00076-5
Arca HC, Mosquera-Giraldo LI, Bi V et al (2018) Pharmaceutical applications of cellulose ethers and cellulose ether Esters. Biomacromol 19:2351–2376. https://doi.org/10.1021/acs.biomac.8b00517
Berlin P, Klemm D, Tiller J, Rieseler R (2000) A novel soluble aminocellulose derivative type: its transparent film-forming properties and its efficient coupling with enzyme proteins for biosensors. Macromol Chem Phys 201:2070–2082. https://doi.org/10.1002/1521-3935(20001001)201:15%3c2070. (:AID-MACP2070>3.0.CO;2-E)
Bernkop-Schnürch A, Dünnhaupt S (2012) Chitosan-based drug delivery systems. Eur J Pharm Biopharm 81:463–469. https://doi.org/10.1016/J.EJPB.2012.04.007
Cimecioglu AL, Ball DH, Kaplan DL, Huang SH (1994) Preparation of amylose derivatives selectively modified at c-6. 6-amino-6-deoxyamylose. Macromolecules 27:2917–2922. https://doi.org/10.1021/ma00089a004
Codera V, Edgar KJ, Faijes M, Planas A (2016) Functionalized celluloses with regular substitution pattern by glycosynthase-catalyzed polymerization. Biomacromol 17:1272–1279. https://doi.org/10.1021/acs.biomac.5b01453
Dong Y, Edgar KJ (2015) Imparting functional variety to cellulose ethers via olefin cross-metathesis. Polym Chem 6:3816–3827
Dong Y, Mosquera-Giraldo LI, Taylor LS, Edgar KJ (2016a) Amphiphilic cellulose ethers designed for amorphous solid dispersion via olefin cross-metathesis. Biomacromol 17:454–465. https://doi.org/10.1021/acs.biomac.5b01336
Dong Y, Mosquera-Giraldo LI, Troutman J et al (2016b) Amphiphilic hydroxyalkyl cellulose derivatives for amorphous solid dispersion prepared by olefin cross-metathesis. Polym Chem 7:4953–4963. https://doi.org/10.1039/c6py00960c
Dong Y, Matson JB, Edgar KJ (2017) Olefin cross-metathesis in polymer and polysaccharide chemistry: a review. Biomacromol 18:1661–1676. https://doi.org/10.1021/acs.biomac.7b00364
Dong Y, Mosquera-Giraldo LI, Taylor LS, Edgar KJ (2017b) Tandem modification of amphiphilic cellulose ethers for amorphous solid dispersion via olefin cross-metathesis and thiol-Michael addition. Polym Chem 8:3129–3139. https://doi.org/10.1039/C7PY00228A
Edgar KJ, Buchanan CM, Debenham JS et al (2001) Advances in cellulose ester performance and application. Prog Polym Sci 26:1605–1688. https://doi.org/10.1016/S0079-6700(01)00027-2
Eissa AM, Khosravi E, Cimecioglu AL (2012) A versatile method for functionalization and grafting of 2-hydroxyethyl cellulose (HEC) via click chemistry. Carbohydr Polym 90:859–869. https://doi.org/10.1016/j.carbpol.2012.06.012
Evano G, Nitelet A, Thilmany P, Dewez DF (2018) Metal-mediated halogen exchange in aryl and vinyl halides: a review. Front Chem 6:114
Evans ME, Long L, Parrish FW (1968) Reaction of carbohydrates with methylsulfonyl chloride in N,N-dimethylformamide. Preparation of some methyl 6-chloro-6-deoxyglycosides. J Org Chem 33:1074–1076. https://doi.org/10.1021/jo01267a028
Fox SC, Edgar KJ (2012) Staudinger reduction chemistry of cellulose: synthesis of selectively O-acylated 6-amino-6-deoxy-cellulose. Biomacromolecules 13:992–1001. https://doi.org/10.1021/bm2017004
Fox SC, Li B, Xu D, Edgar KJ (2011) Regioselective esterification and etherification of cellulose: a review. Biomacromol 12:1956–1972. https://doi.org/10.1021/bm200260d
Furuhata K, Koganei K, Chang H-S et al (1992) Dissolution of cellulose in lithium bromide-organic solvent systems and homogeneous bromination of cellulose with N-bromosuccinimide-triphenylphosphine in lithium bromide-N,N-dimethylacetamide. Carbohydr Res 230:165–177. https://doi.org/10.1016/S0008-6215(00)90519-6
Gao C, Edgar KJ (2019) Efficient synthesis of glycosaminoglycan analogs. Biomacromol 20:608–617. https://doi.org/10.1021/acs.biomac.8b01150
Gao C, Liu S, Edgar KJ (2018) Regioselective chlorination of cellulose esters by methanesulfonyl chloride. Carbohydr Polym 193:108–118. https://doi.org/10.1016/J.CARBPOL.2018.03.093
Gao C, Fisher ZB, Edgar KJ (2019) Azide reduction by DTT or thioacetic acid provides access to amino and amido polysaccharides. Cellul 26:445–462. https://doi.org/10.1007/s10570-018-2195-3
Gericke M, Liebert T, Heinze T (2009) Polyelectrolyte synthesis and in situ complex formation in ionic liquids. J Am Chem Soc 131:13220–13221
Heinze T, Liebert T (2001) Unconventional methods in cellulose functionalization. Prog Polym Sci 26:1689–1762. https://doi.org/10.1016/S0079-6700(01)00022-3
Heinze T, Liebert T, Andreas Koschella (2006) Esterification of polysaccharides. Springer, Cham
Joubert F, Yeo RP, Sharples GJ et al (2015) Preparation of an antibacterial poly(ionic liquid) graft copolymer of hydroxyethyl cellulose. Biomacromol 16:3970–3979. https://doi.org/10.1021/acs.biomac.5b01300
Klemm D, Heublein B, Fink H-PH, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed 44:3358–3393. https://doi.org/10.1002/anie.200460587
Knaus S, Mais U, Binder WH (2003) Synthesis, characterization and properties of methylaminocellulose. Cellulose 10:139–150. https://doi.org/10.1023/A:1024024402735
Kumar MNVR (2000) A review of chitin and chitosan applications. React Funct Polym 46:1–27
Li F, Fei P, Cheng B et al (2019) Synthesis, characterization and excellent antibacterial property of cellulose acetate reverse osmosis membrane via a two-step reaction. Carbohydr Polym 216:312–321. https://doi.org/10.1016/j.carbpol.2019.04.026
Liebert T, Hänsch C, Heinze T (2006) Click chemistry with polysaccharides. Macromol Rapid Commun 27:208–213. https://doi.org/10.1002/marc.200500686
Liu C, Baumann H (2002) Exclusive and complete introduction of amino groups and their N-sulfo and N-carboxymethyl groups into the 6-position of cellulose without the use of protecting groups. Carbohydr Res 337:1297–1307. https://doi.org/10.1016/S0008-6215(02)00132-5
Liu C, Baumann H (2005) New 6-butylamino-6-deoxycellulose and 6-deoxy-6-pyridiniumcellulose derivatives with highest regioselectivity and completeness of reaction. Carbohydr Res 340:2229–2235. https://doi.org/10.1016/J.CARRES.2005.07.018
Liu S, Edgar KJ (2017) Water-soluble co-polyelectrolytes by selective modification of cellulose esters. Carbohydr Polym 162:1–9. https://doi.org/10.1016/J.CARBPOL.2017.01.008
Liu S, Liu J, Esker AR, Edgar KJ (2016) An efficient, regioselective pathway to cationic and zwitterionic N -heterocyclic cellulose ionomers. Biomacromol 17:503–513. https://doi.org/10.1021/acs.biomac.5b01416
Liu S, Gao C, Mosquera-Giraldo LI et al (2018) Selective synthesis of curdlan ω-carboxyamides by staudinger ylide nucleophilic ring-opening. Carbohydr Polym 190:222–231. https://doi.org/10.1016/J.CARBPOL.2018.02.074
Matsui Y, Ishikawa J, Kamitakahara H et al (2005) Facile synthesis of 6-amino-6-deoxycellulose. Carbohydr Res 340:1403–1406. https://doi.org/10.1016/J.CARRES.2005.02.030
McCormick CL, Dawsey TR, Newman JK (1990) Competitive formation of cellulose p-toluenesulfonate and chlorodeoxycellulose during homogeneous reaction of p-toluenesulfonyl chloride with cellulose in N,N-dimethylacetamide-lithium chloride. Carbohydr Res 208:183–191. https://doi.org/10.1016/0008-6215(90)80098-N
McNamara JT, Morgan JLW, Zimmer J (2015) A molecular description of cellulose biosynthesis. Ann Rev Biochem 84:895–921. https://doi.org/10.1146/annurev-biochem-060614-033930
Meng X, Edgar KJ (2016) Click reactions in polysaccharide modification. Prog Polym Sci 53:52–85. https://doi.org/10.1016/J.PROGPOLYMSCI.2015.07.006
Mischnick P, Momcilovic D (2010) Chemical structure analysis of starch and cellulose derivatives. Advances in carbohydrate chemistry and biochemistry. Academic Press, Cambridge, pp 117–210
Mischnick P, Niedner W, Adden R (2005) Possibilities of mass spectrometry and tandem-mass spectrometry in the analysis of cellulose ethers. Macromol Symp 223:67–78. https://doi.org/10.1002/masy.200550505
Mosquera-Giraldo LI, Borca CH, Meng X et al (2016) Mechanistic design of chemically diverse polymers with applications in oral drug delivery. Biomacromolecules 17:3659–3671. https://doi.org/10.1021/acs.biomac.6b01156
Nichols BLB, Chen J, Mischnick P, Edgar KJ (2020) Selective oxidation of 2-hydroxypropyl ethers of cellulose and dextran: simple and efficient introduction of versatile ketone groups to polysaccharides. Biomacromolecules. https://doi.org/10.1021/acs.biomac.0c01045. (Biomacromolecules 21)
Nikolaeva D, Verachtert K, Azcune I et al (2021) Influence of ionic liquid-like cationic pendants composition in cellulose based polyelectrolytes on membrane-based CO2 separation. Carbohydr Polym 255:117375. https://doi.org/10.1016/J.CARBPOL.2020.117375
Petzold-Welcke K, Michaelis N, Heinze T et al (2009) Unconventional cellulose products through nucleophilic displacement reactions. Macromol Symp 280:72–85. https://doi.org/10.1002/masy.200950609
Pollak PI, Curtin DY (1950) Stereospecificity in the rearrangement of Amino Alcohols. J Am Chem Soc 72:961–965. https://doi.org/10.1021/JA01158A082/ASSET/JA01158A082.FP.PNG_V03
Reinhart K, Hartog CS (2012) Hydroxyethyl starch in patients with trauma. Br J Anaesth 108:321–322. https://doi.org/10.1093/bja/aer467
Ren J, Wang P, Dong F et al (2012) Synthesis and antifungal properties of 6-amino-6-deoxyinulin, a kind of precursors for facile chemical modifications of inulin. Carbohydr Polym 87:1744–1748. https://doi.org/10.1016/J.CARBPOL.2011.09.082
Sato T, Koizumi J, Ohno Y, Endo T (1990) An improved procedure for the preparation of chlorinated cellulose with methanesulfonyl chloride in a dimethylformamide–chloral–pyridine mixture. J Polym Sci A Polym Chem 28:2223–2227. https://doi.org/10.1002/pola.1990.080280817
Schmidt S, Liebert T, Heinze T (2014) Synthesis of soluble cellulose tosylates in an eco-friendly medium. Green Chem 16:1941–1946. https://doi.org/10.1039/C3GC41994K
Svensson A, Sjöström J, Scheel T, Piculell L (2003) Phases and structures of a polyion-surfactant ion complex salt in aqueous mixtures: cationic hydroxyethyl cellulose with dodecylsulfate counterions. Coll Surf A Physicochem Eng Asp 228:91–106. https://doi.org/10.1016/S0927-7757(03)00377-7
Wang X, Wu M, Zhang B et al (2019) Phase-transfer method synthesis hydroxyethyl cellulose lauryl ether. Coll Surf A Physicochem Eng Asp 562:383–391. https://doi.org/10.1016/j.colsurfa.2018.11.055
Wang K, Ye L (2010) Structure and property of cationic hydroxyethyl cellulose. Polym Plast Technol Eng 49:807–811. https://doi.org/10.1080/03602551003749619
Winstein S, Holness NJ (1955) Neighboring carbon and hydrogen. XIX. t-butylcyclohexyl derivatives. quantitative conformational analysis. J Am Chem Soc 77:5562–5578. https://doi.org/10.1021/JA01626A037/ASSET/JA01626A037.FP.PNG_V03
Zhang R, Edgar KJ (2014) Synthesis of curdlan derivatives regioselectively modified at C-6: O–(N)-Acylated 6-amino-6-deoxycurdlan. Carbohydr Polym 105:161–168. https://doi.org/10.1016/J.CARBPOL.2014.01.045
Zhang R, Edgar KJ (2015) Water-soluble aminocurdlan derivatives by chemoselective azide reduction using NaBH 4. Carbohydr Polym 122:84–92. https://doi.org/10.1016/J.CARBPOL.2014.12.020
Zhang R, Liu S, Edgar KJ (2017) Efficient synthesis of secondary amines by reductive amination of curdlan Staudinger ylides. Carbohydr Polym 171:1–8. https://doi.org/10.1016/J.CARBPOL.2017.04.093
Zhou S, Xu C, Wang J et al (2004) Phase Behavior of cationic hydroxyethyl cellulose – sodium dodecyl sulfate mixtures: effects of molecular weight and ethylene oxide side chain length of polymers. Langmuir 20:8482–8489. https://doi.org/10.1021/la049142n
Acknowledgments
We thank the Institute for Critical Technology and Applied Science (ICTAS), the Macromolecules Innovation Institute (MII), and the Virginia Tech Departments of Sustainable Biomaterials and of Chemistry for their financial, facilities, and educational support. We thank Dr. Xiuli Li for providing insights regarding NMR data interpretation.
Funding
We gratefully acknowledge partial funding support of the work from the National Science Foundation through grant IIP-1827493 (SP), and through grant DMR-2204996 (KJE). This work was partially supported by GlycoMIP, a National Science Foundation Materials Innovation Platform funded through Cooperative Agreement DMR-1933525.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Synthesis, characterization, and data interpretation and analysis, were performed by CG and SP. The first draft of the manuscript was written by CG and all authors contributed to the editing of the manuscript, including preparation of the final version. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, C., Petrova, S.P. & Edgar, K.J. Chlorination of hydroxyethyl cellulose enables selective functionalization. Cellulose 31, 1481–1495 (2024). https://doi.org/10.1007/s10570-023-05675-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05675-x