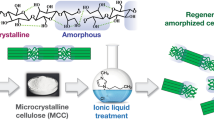
Abstract
The main objective of this work is to present a comprehensive analysis of the morphology, topography, and thermal behavior of modified cellulose as a function of solvation temperature, ionic liquid type, and regeneration agent. Avicel microcrystalline cellulose was dissolved at two temperatures (73 °C and 100 °C) in two competing imidazolium-based ionic liquids (1-ethyl-3-methylimidazolium acetate and 1-ethyl-3-methylimidazolium bromide). Aliquots of each solvent-polymer mixture were then washed and regenerated in competing baths (D.I. water and 25% hydrogen peroxide solution). A comparative analysis of the work is that the solvation temperature and ionic liquid counterion (AcO− and Br−) had direct relationships to polymer solubility and physicochemical properties of the cellulose samples. Also, treatment of hydrogen peroxide solution as a regenerative agent influenced crystal sizes within the samples. Partial dissolution of cellulose induced by 1-ethyl-3-methylimidazolium bromide influenced the production of unique morphological changes and thermal behaviors relative to microcrystalline cellulose and other cellulose preparations.




Similar content being viewed by others
Availability of data and materials
Supplementary information to this work is available as an online resource.
References
Astruc J, Nagalakshmaiah M, Laroche G, Grandbois M, Elkoun S, Robert M (2017) Isolation of cellulose-II nanospheres from flax stems and their physical and morphological properties. Carbohydr Polym 178:352–359
Blessing B, Trout C, Morales A, Rybacki K, Love SA, Lamoureux G, Salas-de la Cruz D (2019) Morphology and ionic conductivity relationship in silk/cellulose biocomposites. Polym Int 68(9):1580–1590
Cao Y, Zhang R, Cheng T, Guo J, Xian M, Liu H (2017) Imidazolium-based ionic liquids for cellulose pretreatment: recent progresses and future perspectives. Appl Microbiol Biotechnol 101(2):521–532
Cheng G, Varanasi P, Li C, Liu H, Melnichenko YB, Simmons BA, Singh S (2011) Transition of cellulose crystalline structure and surface morphology of biomass as a function of ionic liquid pretreatment and its relation to enzymatic hydrolysis. Biomacromol 12(4):933–941
Crawford B, Ismail AE (2020) Insight into cellulose dissolution with the tetrabutylphosphonium chloride–water mixture using molecular dynamics simulations. Polymers 12(3):627
Das K, Ray D, Bandyopadhyay NR, Sengupta S (2010) Study of the properties of microcrystalline cellulose particles from different renewable resources by XRD, FTIR, nanoindentation, TGA and SEM. J Polym Environ 18:355–363
French AD (2014) Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 21(2):885–896
Hadadi A, Whittaker JW, Verrill DE, Hu X, Larini L, Salas-De La Cruz D (2018) A hierarchical model to understand the processing of polysaccharides/protein-based films in ionic liquids. Biomacromol 19(10):3970–3982
Helbert W, Nishiyama Y, Okano T, Sugiyama J (1998) Molecular imaging of halocynthia papillosa cellulose. J Struct Biol 124(1):42–50
Ishikawa A, Okano T, Sugiyama J (1997) Fine structure and tensile properties of ramie fibres in the crystalline form of cellulose I, II IIII and IVI. Polymer 38(2):463–468
Johnson KE (2007) What’s an ionic liquid? Electrochem Soc Interface 16(1):38
Jones OG, McClements DJ (2010) Functional biopolymer particles: design, fabrication, and applications. Compr Rev Food Sci Food Saf 9(4):374–397
Kavkler K, Gunde-Cimerman N, Zalar P, Demšar A (2011) FTIR spectroscopy of biodegraded historical textiles. Polym Degrad Stab 96(4):574–580
Khan T, Park JK, Kwon JH (2007) Functional biopolymers produced by biochemical technology considering applications in food engineering. Korean J Chem Eng 24:816–826
Li Y, Lin M, Davenport JW (2011) Ab initio studies of cellulose I: crystal structure, intermolecular forces, and interactions with water. J Phys Chem C 115(23):11533–11539
Liu Z, Wang H, Li Z, Lu X, Zhang X, Zhang S, Zhou K (2011) Characterization of the regenerated cellulose films in ionic liquids and rheological properties of the solutions. Mater Chem Phys 128(1–2):220–227
Love SA, Popov E, Rybacki K, Hu X, Salas-de la Cruz D (2020) Facile treatment to fine-tune cellulose crystals in cellulose-silk biocomposites through hydrogen peroxide. Int J Biol Macromol 147:569–575
Medronho B, Lindman B (2015) Brief overview on cellulose dissolution/regeneration interactions and mechanisms. Adv Colloids Interface Sci 222:502–508
Metzke M, Guan Z (2008) Structure-property studies on carbohydrate-derived polymers for use as protein-resistant biomaterials. Biomacromol 9(1):208–215
Mohd N, Draman SFS, Salleh MSN, Yusof NB (2017) Dissolution of cellulose in ionic liquid: a review. In: AIP conference proceedings (Vol. 1809, No. 1, p. 020035). AIP Publishing LLC
Perez S, Samain D (2010) Structure and engineering of celluloses. Adv Carbohydr Chem Biochem 64:25–116
Rhim JW, Ng PK (2007) Natural biopolymer-based nanocomposite films for packaging applications. Crit Rev Food Sci Nutr 47(4):411–433
Song R, Murphy M, Li C, Ting K, Soo C, Zheng Z (2018) Current development of biodegradable polymeric materials for biomedical applications. Drug Des Dev Ther 12:3117–3145
Stanton J, Xue Y, Waters JC, Lewis A, Cowan D, Hu X, la Cruz DSD (2017) Structure–property relationships of blended polysaccharide and protein biomaterials in ionic liquid. Cellulose 24:1775–1789
Stanton J, Xue Y, Pandher P, Malek L, Brown T, Hu X, Salas-de la Cruz D (2018) Impact of ionic liquid type on the structure, morphology and properties of silk-cellulose biocomposite materials. Int J Biol Macromol 108:333–341
Sun X, Tian Q, Xue Z, Zhang Y, Mu T (2014) The dissolution behaviour of chitosan in acetate-based ionic liquids and their interactions: From experimental evidence to density functional theory analysis. RSC Adv 4(57):30282–30291
Wang H, Gurau G, Rogers RD (2012) Ionic liquid processing of cellulose. Chem Soc Rev 41(4):1519–1537
Yin J, Luan S (2016) Opportunities and challenges for the development of polymer-based biomaterials and medical devices. Regen Biomater 3(2):129–135
Zhang H, Wu J, Zhang J, He J (2005) 1-Allyl-3-methylimidazolium chloride room temperature ionic liquid: a new and powerful nonderivatizing solvent for cellulose. Macromolecules 38(20):8272–8277
Zhao D, Li H, Zhang J, Fu L, Liu M, Fu J, Ren P (2012) Dissolution of cellulose in phosphate-based ionic liquids. Carbohydr Polym 87(2):1490–1494
Acknowledgments
A special thank you for the acquisition of x-ray data by Abneris Morales, Ph.D. student of The Center for Computational and Integrative Biology at Rutgers University–Camden and for technical support and training on the DEXS system by Dr. Paul Heiney, emeritus professor for the Department of Physics and Astronomy at the University of Pennsylvania and supervisor/coordinator for the DEXS facility. Salas-de la Cruz summer financial support was provided by NSF-CMMI (2037097) and NSF-DMR (2104376).
Funding
The authors are grateful for financial support from NSF-DMR-RUI 1809354 and NSF-CMMI 2037097, Rutgers-Camden Arts and Sciences Start-up Package, State of NJ ELF Grant and The Center for Computational and Integrative Biology TA Funds. The DEXS System is supported by NSF-MRSEC 17-20530, NSF-MRI 17-25969, ARO DURIP W911NF-17-1-02822, and the University of Pennsylvania.
Author information
Authors and Affiliations
Contributions
Conceptualization: SAL and DS-de laC; Methodology: DS-de laC and SAL; Formal analysis and investigation: SAL, EM, DSM, and SS; Writing—original draft preparation: SAL, EM, DSM, and SS; Writing—review and editing: SAL and DS-de laC; Funding acquisition: DS-de laC; Resources: DS-de laC; Supervision: DS-de laC and SAL.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval and consent to participate
The authors declare no sensitive or personal information is reported within this work.
Consent for publication
The authors consent to the publication of this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Love, S.A., Magaziner, E., Melissaratos, D.S. et al. Cellulose solubility in ionic liquids and regeneration in water and hydrogen peroxide solution: a comparative examination of morphology and physicochemical properties. Cellulose 30, 6149–6162 (2023). https://doi.org/10.1007/s10570-023-05258-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-023-05258-w