Abstract
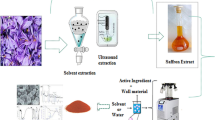
In order to provide stability of anthocyanin, a powerful antioxidant, encapsulant was made by utilizing two biopolymers that can be used further for the targeted release due to its intrinsic structure. Present study was carried out to encapsulate anthocyanin in coacervate employing pectin and chitosan. Entrapment efficiency of anthocyanin was evaluated in coacervates prepared in nine different combinations employing three concentration each of pectin (40, 50 and 60 mg mL−1) and chitosan (5, 6 and 7 mg mL−1) in presence of CaCl2. The developed formulation was characterised by FT-IR, XRD and SEM techniques, which clearly depicts ionic interaction between chitosan and pectin. Release pattern of anthocyanin from the coacervate followed Korsmeyer-Peppas model. The n-values calculated from the curves suggested a quasi-Fickian diffusion pattern pH 1.7 and 4.0. However, at pH 7.0, the behaviour of release was presumed to be an anomalous or case-II transport. In-vitro study revealed that bioaccessibility (%) of coacervated formulation (24.3 ± 0.5) was significantly higher than crude extract (12.2 ± 1.0) as well as purified anthocyanin crystals (18.9 ± 0.7). Better bioaccessibility of coacervated anthocyanin provided important information about its potential use in functional foods.






Similar content being viewed by others
References
Al-Azi SOSM, Tan YTF, Wong TW (2014) Transforming large molecular weight pectin and chitosan into oral protein drug nanoparticulate carrier. React Funct Polym 84:45–52
Andishmand H, Tabibiazar M, Mohammadifar MA et al (2017) Pectin-zinc-chitosan-polyethylene glycol colloidal nano-suspension as a food grade carrier for colon targeted delivery of resveratrol. Int J Biol Macromol 97:16–22
Bernabé P, Peniche C, Argüelles-Monal W (2005) Swelling behavior of chitosan/pectin polyelectrolyte complex membranes. Effect of thermal cross-linking. Polymer Bull 55:367–375
Bigucci F, Luppi B, Cerchiara T et al (2008) Chitosan/pectin polyelectrolyte complexes: selection of suitable preparative conditions for colon-specific delivery of vancomycin. Eur J Pharm Sci 35:435–441
Bouayed J, Hoffmann L, Bohn T (2011) Total phenolics, flavonoids, anthocyanins and antioxidant activity following simulated gastro-intestinal digestion and dialysis of apple varieties: bioaccessibility and potential uptake. Food Chem 128:14–21
Boyapally H, Nukala RK, Bhujbal P et al (2010) Controlled release from directly compressible theophylline buccal tablets. Coll Surf B Biointerfaces 77(2):227–233
Carrillo C, Buvé C, Panozzo A et al (2017) Role of structural barriers in the in vitro bioaccessibility of anthocyanins in comparison with carotenoids. Food Chem 227:271–279
Comunian TA, Thomazini M, Alves AJG et al (2013) Microencapsulation of ascorbic acid by complex coacervation: protection and controlled release. Food Res Int 52:373–379
Dall'Asta M, Calani L, Tedeschi M et al (2012) Identification of microbial metabolites derived from in vitro fecal fermentation of different polyphenolic food sources. Nutrition 28(2):197–203
de Souza JRR, de Carvalho JIX, Trevisan MTS et al (2009) Chitosan-coated pectin beads: characterization and in vitro release of mangiferin. Food Hydrocoll 23:2278–2286
De Yao K, Liu J, Cheng GX et al (1996) Swelling behavior of pectin/chitosan complex films. J Appl Polym Sci 60:279–283
Fernández-Hervása MJ, Fellb JT (1998) Pectin/chitosan mixtures as coatings for colon-specific drug delivery: an in vitro evaluation. Int J Pharm 169:115–119
Ghaffari A, Navaee K, Oskoui M et al ( (2007) Preparation and characterization of free mixed-film of pectin/chitosan/Eudragit®RS intended for sigmoidal drug delivery. Eur J Pharm Biopharm 67:175–186 )
Gil-Izquierdo A, Zafrilla P, Tomás-Barberán (2002) An in vitro method to simulate phenolic compound release from the food matrix in the gastrointestinal tract. Eur Food Res Tech 214:155–159
Huang GQ, Liu LN, Han XN et al (2017) Intestine-targeted delivery potency of the O-carboxymethyl chitosan–gum Arabic coacervate: Effects of coacervation acidity and possible mechanism. Materials Sci Eng C 79:423–429
Kamiloglu S, Capanoglu E, Grootaert C et al (2015) Anthocyanin absorption and metabolism by human intestinal Caco-2 cells—a review. Int J Mol Sci 16:21555–21574
Kim TH, Park YH, Kim KJ et al (2003) Release of albumin from chitosan-coated pectin beads in vitro. Int J Pharm 250:371–383
Konczak I, Zhang W (2004) Anthocyanins—more than nature’s colours. J Biomed Biotech 239–240
Kurukji D, Norton I, Spyropoulos F (2016) Fabrication of sub-micron protein-chitosan electrostatic complexes for encapsulation and pH-modulated delivery of model hydrophilic active compounds. Food Hydrocoll 53:249–260
Liang L, Wu X, Zhao T et al (2012) In vitro bioaccessibility and antioxidant activity of anthocyanins from mulberry (Morus atropurpurea Roxb.) following simulated gastro-intestinal digestion. Food Res Int 46:76–82
McDougall GJ, Dobson P, Smith P et al (2005) Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. J Agric Food Chem 53:5896–5904
Mülleder U, Murkovic M, Pfannhauser W (2002) Urinary excretion of cyanidin glycosides. J Biochem Biophys Methods 53:61–66
Naidu VGM, Madhusudhana K, Sashidhar RB et al (2009) Polyelectrolyte complexes of gum kondagogu and chitosan, as diclofenac carriers. Carbohydr Polym 76(3):464–471
Nori MP, Favaro-Trindade CS, Matias de Alencar S et al (2011) Microencapsulation of propolis extract by complex coacervation. LWT-Food Sci Technol 44:429–435
Oidtmann J, Schantz M, Mäder K et al (2012) Preparation and comparative release characteristics of three anthocyanin encapsulation systems. J Agric Food Chem 60:844–851
Pandey S, Mishra A, Raval P et al (2013) Chitosan-pectin polyelectrolyte complex as a carrier for colon targeted drug delivery. J Young Pharm 5:160–166
Saha S, Singh J, Paul A et al (2020) Anthocyanin Profiling Using UV-Vis Spectroscopy and Liquid Chromatography Mass Spectrometry. J AOAC Int 103(1):23–39
Sarkar R, Kundu A, Banerjee K et al (2018) Anthocyanin composition and potential bioactivity of karonda (Carissa carandas) L. fruit: an Indian source of biocolorant. LWT-Food Sci Technol 93:673–678
Shaddel R, Hesari J, Azadmard-Damirchi S et al (2018) Double emulsion followed by complex coacervation as a promising method for protection of black raspberry anthocyanins. Food Hydrocoll 77:803–816
Silva DF, Favaro-Trindade CS, Rocha GA et al (2012) Microencapsulation of lycopene by gelatin-pectin complex coacervation. J Food Proc Preserv 36:185–190
Talavera S, Felgines C, Texier O et al (2003) Anthocyanins are efficiently absorbed from the stomach in anesthetized rats. J Nutr 133:4178–4182
Yang M, Koo SI, Song WO et al (2011) Food matrix affecting anthocyanin bioavailability: review. Curr Med Chem 18:291–300
Yao KD, Tu H, Cheng F et al (1997) pH-sensitivity of the swelling of a chitosan-pectin polyelectrolyte complex. Die Angewandte Makromolekulare Chemie 245:63–72
Acknowledgments
Authors are grateful to Head, Division of Agricultural Chemicals for providing all the facilities. Authors are also thankful to ICAR for funding this work. The present work was funded by the Senior Research Fellowship, ICAR, New Delhi.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest among authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material
Rights and permissions
About this article
Cite this article
Sarkar, R., Dutta, A., Patra, A. et al. Bio-inspired biopolymeric coacervation for entrapment and targeted release of anthocyanin. Cellulose 28, 377–388 (2021). https://doi.org/10.1007/s10570-020-03523-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03523-w