Abstract
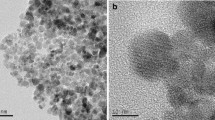
Chitosan grafted titanium dioxide (CS/TiO2) was synthesized to remove U(VI) and Eu(III) from aqueous solutions. The experimental results demonstrated that the removal of radionuclides was dependent on pH. The simultaneous elimination of U(VI) and Eu(III) proved that the presence of Eu(III) competed with U(VI) adsorption and caused the decreased elimination of U(VI) and vice versa. The retention of U(VI) on CS/TiO2 relied on the hydroxyl groups, and the Eu(III) removal could be attributed to the amidogen groups. DFT calculation results demonstrated that the most likely adsorption site was located between the chitosan and titanium dioxide. The adsorption of radionuclides on CS/TiO2 was the combinations of various adsorption situations. This study can contribute to the simultaneous adsorption of radionuclides and provide important information for removal of other contaminants.







Similar content being viewed by others
References
Alam MS, Gorman-Lewis D, Chen N, Safari S, Baek K, Konhauser KO, Alessi DS (2018) Mechanisms of the removal of U(VI) from aqueous solution using biochar: a combined spectroscopic and modeling approach. Environ Sci Technol 52:13057–13067
Arunraj B, Talasila S, Rajesh V, Rajesh N (2019) Removal of europium from aqueous solution using Saccharomyces cerevisiae immobilized in glutaraldehyde cross-linked chitosan. Separ Sci Technol 54:1620–1631
Chen Y, Chen L, Bai H, Li L (2013) Graphene oxide-chitosan composite hydrogels as broad-spectrum adsorbents for water purification. J Mater Chem A 1:1992–2001
Chou JP, Hsing CR, Chen JC, Lee JY, Wei CM (2013a) New structural model for Na6Si3 surface magic cluster on the Si(111)-7 × 7 surface. Surf Sci 616:137–142
Chou JP, Hsing CR, Wei CM, Cheng C, Chang CM (2013b) Ab initio random structure search for 13-atom clusters of fcc elements. J Phys-condens Mat 25(12):125305
Combernoux N, Schrive L, Labed V, Wyart Y, Carretier E, Moulin P (2017) Treatment of radioactive liquid effluents by reverse osmosis membranes: From lab-scale to pilot-scale. Water Res 123:311–320
Deng H, Li ZJ, Wang XC, Wang L, Liu K, Yuan LY, Chang ZY, Gibson JK, Zheng LR, Chai ZF, Shi WQ (2019) Efficient photocatalytic reduction of aqueous perrhenate and pertechnetate. Environ Sci Technol 53:10917–10925
Garcia-Rates M, Lopez N (2016) Multigrid-based methodology for implicit solvation models in periodic DFT. J Chem Theory Comput 12:1331–1341
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104
Haldorai Y, Shim JJ (2014) An efficient removal of methyl orange dye from aqueous solution by adsorption onto chitosan/MgO composite: a novel reusable adsorbent. Appl Surf Sci 292:447–453
He J, Bardelli F, Gehin A, Silvester E, Charlet L (2016) Novel chitosan goethite bionanocomposite beads for arsenic remediation. Water Res 101:1–9
Hettiarachchi E, Paul S, Cadol D, Frey B, Rubasinghege G (2019) Mineralogy controlled dissolution of uranium from airborne dust in simulated lung fluids (SLFs) and possible health implications. Environ Sci Tech Let 6:62–67
Huang ZW, Li ZJ, Wu QY, Zheng LR, Zhou LM, Chai ZF, Wang XL, Shi WQ (2018) Simultaneous elimination of cationic uranium(VI) and anionic rhenium(VII) by graphene oxide-poly(ethyleneimine) macrostructures: a batch, XPS, EXAFS, and DFT combined study. Environ Sci-Nano 5:2077–2087
Imam EA, El-Sayed IET, Mahfouz MG, Tolba AA, Akashi T, Galhoum AA, Guibal E (2018) Synthesis of alpha-aminophosphonate functionalized chitosan sorbents: effect of methyl vs phenyl group on uranium sorption. Chem Eng J 352:1022–1034
Kahu SS, Shekhawat A, Saravanan D, Jugade RM (2016) Two fold modified chitosan for enhanced adsorption of hexavalent chromium from simulated wastewater and industrial effluents. Carbohyd Polym 146:264–273
Kang D, Yu X, Ge M, Xiao F, Xu H (2017) Novel Al-doped carbon nanotubes with adsorption and coagulation promotion for organic pollutant removal. J Environ Sci 54:1–12
Kresse G (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54(16):11169–11186
Kresse G, Furthmüller J (1996) Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comp mater Sci 6(1):15–50
Lim J, Monllor-Satoca D, Jang JS, Lee S, Choi W (2014) Visible light photocatalysis of fullerol-complexed TiO2 enhanced by Nb doping. Appl Catal B-Environ 152:233–240
Liu H, Chou JP, Li RW, Wei CM, Miki K (2011) Trimeric precursors in formation of Al magic clusters on a Si(111)-7 × 7 surface. Phys Rev B 83(7):401–406
Ma S, Huang L, Ma L, Shim Y, Islam SM, Wang P, Zhao LD, Wang S, Sun G, Yang X, Kanatzidis M (2015) Efficient uranium capture by polysulfide/layered double hydroxide composites. J Am Chem Soc 137:3670–3677
Manos MJ, Kanatzidis MG (2012) Layered metal sulfides capture uranium from seawater. J Am Chem Soc 134:16441–16446
Mei L, Li FZ, Lan JH, Wang CZ, Xu C, Deng H, Wu QY, Hu KQ, Wang L, Chai ZF, Chen J, Gibson J, Shi WQ (2019) Anion-adaptive crystalline cationic material for 99TcO4– trapping. Nat Commun 10:1532
Modrzejewska Z, Sujka W, Dorabialska M, Zarzycki R (2006) Adsorption of Cr(VI) on cross-linked chitosan beads. Separ Sci Technol 41:111–122
Murakami M, Shibayama N, Sueki K, Mouri G, O H, Nomura M, Koibuchi Y, Oki T (2016) Occurrence and partition ratios of radiocesium in an urban river during dry and wet weather after the 2011 nuclear accident in Fukushima. Water Res 92:87–93
Pang H, Diao Z, Wang X, Ma Y, Yu S, Zhu H, Chen Z, Hu B, Chen J, Wang X (2019) Adsorptive and reductive removal of U(VI) by dictyophora indusiate-derived biochar supported sulfide NZVI from wastewater. Chem Eng J 366:368–377
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys rev let 77(18):3865
Sadergaski LR, Stoxen W, Hixon AE (2018) Uranyl peroxide nanocluster (U-60) persistence and sorption in the presence of hematite. Environ Sci Technol 52:3304–3311
Shang H, Guo H, Ma C, Li C, Chefetz B, Polubesova T, Xing B (2019) Maize (Zea mays L.) root exudates modify the surface chemistry of CuO nanoparticles: Altered aggregation, dissolution and toxicity. Sci Total Environ 690:502–510
Tan X, Fang M, Li J, Lu Y, Wang X (2009) Adsorption of Eu(III) onto TiO2: effect of pH, concentration, ionic strength and soil fulvic acid. J Hazard Mater 168:458–465
Wang JH, Zheng SR, Liu JL, Xu ZY (2010) Tannic acid adsorption on amino-functionalized magnetic mesoporous silica. Chem Eng J 165:10–16
Wang J, Yao W, Gu P, Yu S, Wang X, Du Y, Wang H, Chen Z, Hayat T, Wang X (2017) Efficient coagulation of graphene oxide on chitosan–metal oxide composites from aqueous solutions. Cellulose 24:851–861
Wang L, Song H, Yuan L, Li Z, Zhang Y, Gibson JK, Zheng L, Chai Z, Shi W (2018a) Efficient U(VI) reduction and sequestration by Ti2CTx MXene. Environ Sci Technol 52:10748–10756
Wang Z, Brown AT, Tan K, Chabal YJ, Balkus KJ Jr (2018b) Selective extraction of thorium from rare earth elements using wrinkled mesoporous carbon. J Am Chem Soc 140:14735–14739
Wang JJ, He BH, Wei XY, Li P, Liang JJ, Qiang SR, Fan QH, Wu WS (2019a) Sorption of uranyl ions on TiO2: effects of pH, contact time, ionic strength, temperature and HA. J Environ Sci 75:115–123
Wang J, Zhang R, Huo Y, Ai Y, Gu P, Wang X, Li Q, Yu S, Chen Y, Yu Z, Chen J, Wang X (2019b) Efficient elimination of Cr(VI) from aqueous solutions using sodium dodecyl sulfate intercalated molybdenum disulfide. Ecotox Environ Safe 175:251–262
Wang J, Zhu M, Chen Z, Chen Y, Hayat T, Alsaedi A, Wang X (2019c) Polyacrylamide modified molybdenum disulfide composites for efficient removal of graphene oxide from aqueous solutions. Chem Eng J 361:651–659
Wang L, Song H, Yuan L, Li Z, Zhang P, Gibson JK, Zheng L, Wang H, Chai Z, Shi W (2019d) Effective removal of anionic Re(VII) by surface-modified Ti2CTx MXene nanocomposites: implications for Tc(VII) sequestration. Environ Sci Technol 53:3739–3747
Wang X, Li X, Wang J, Hongtao Z (2020) Recent advances in carbon nitride-based nanomaterials for the removal of heavy metal ions from aqueous solution. J Inorg Mater 35:260–270
Wazne M, Meng X, Korfiatis GP, Christodoulatos C (2006) Carbonate effects on hexavalent uranium removal from water by nanocrystalline titanium dioxide. J Hazard Mater 136:47–52
Yao W, Wu Y, Pang H, Wang X, Yu S, Wang X (2018) In-situ reduction synthesis of manganese dioxide@polypyrrole core/shell nanomaterial for highly efficient enrichment of U(VI) and Eu(III). Sci China Chem 61:812–823
Yin L, Hu Y, Ma R, Wen T, Wang X, Hu B, Yu Z, Hayat T, Alsaedi A, Wang X (2019) Smart construction of mesoporous carbon templated hierarchical Mg-Al and Ni-Al layered double hydroxides for remarkably enhanced U(VI) management. Chem Eng J 359:1550–1562
Younes AA, Masoud AM, Taha MH (2018) Uranium sorption from aqueous solutions using polyacrylamide-based chelating sorbents. Separ Sci Technol 53:2573–2586
Yu J, Yuan L, Wang S, Lan J, Zheng L, Xu C, Chen J, Wang L, Huang Z, Tao W, Liu Z, Chai Z, Gibson JK, Shi W (2019) Phosphonate-decorated covalent organic frameworks for actinide extraction: a breakthrough under highly acidic conditions. CCS Chemistry 1:286–295
Yu X, Tong S, Ge M, Zuo J, Cao C, Song W (2013) One-step synthesis of magnetic composites of cellulose@iron oxide nanoparticles for arsenic removal. J Mater Chem A 1:959–965
Yuan L, Tian M, Lan J, Cao X, Wang X, Chai Z, Gibson JK, Shi W (2018) Defect engineering in metal-organic frameworks: a new strategy to develop applicable actinide sorbents. Chem Commun 54:370–373
Zhang J, Deng Y, Gu D, Wang S, She L, Che R, Wang ZS, Tu B, Xie S, Zhao D (2011) Ligand-assisted assembly approach to synthesize large-pore ordered mesoporous titania with thermally stable and crystalline framework. Adv Energy Mater 1:241–248
Zhang C, Liu X, Tinnacher RM, Tournassat C (2018) Mechanistic understanding of uranyl ion complexation on montmorillonite edges: a combined first-principles molecular dynamics-surface complexation modeling approach. Environ Sci Technol 52:8501–8509
Zheng J, Tagami K, Bu W, Uchida S, Watanabe Y, Kubota Y, Fuma S, Ihara S (2014) 135Cs/137Cs isotopic ratio as a new tracer of radiocesium released from the fukushima nuclear accident. Environ Sci Technol 48:5433–5438
Acknowledgments
This work was supported by the National Natural Science Foundation of China (21777039), the National Key Research and Development Program of China (2017YFA0207002), the China Postdoctoral Science Foundation (2018M641293), the Fundamental Research Funds for the Central Universities (2017YQ001), and the MOE Key Laboratory of Resources and Environmental Systems Optimization (NCEPU). The simulations were performed on resources provided by the Swedish National Infrastructure for Computing (SNIC) at [SNIC CENTER].
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, J., Huo, Y. & Ai, Y. Experimental and theoretical studies of chitosan modified titanium dioxide composites for uranium and europium removal. Cellulose 27, 7765–7777 (2020). https://doi.org/10.1007/s10570-020-03337-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03337-w