Abstract
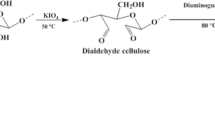
This work was an attempt to prepare a new eco-friendly adsorbent for the recovery of uranium from the Egyptian radioactive deposits. So, cellulose/p-toluidine was prepared as a potential sorbent for uranium (VI) in highly alkaline solutions, from a condensation reaction of p-toluene with dialdehyde cellulose which resulting from periodate selective oxidative of cellulose. We have studied the kinetics, isotherm models, and thermodynamic of uranium adsorption from aqueous solutions by dissolved the deposits uranium in sodium carbonate aqueous solution. The effect of adsorption time, and temperature, pH, as well as uranium concentration on adsorption capacity were investigated. The adsorption capacity of uranium by cellulose p-toluidine has been found to attain 80 mg/g. Scanning electron microscopy, X-ray diffractometer, and Fourier transform infrared spectroscopy were used to study the morphological characterization, and chemical structure of the prepared adsorbent. In addition, the computational calculation of dialdehyde cellulose, p-toluidine, and cellulose/p-toluidine by DFT/B3LYP/6-31G (d) basis sets was studied. The adsorption of uranium using cellulose p-toluidine followed fitted with pseudo-second-order reaction as well as Langmuir isotherm. Also, the prepared adsorbent displayed excellent antimicrobial activity against Staphylococcus aureus, Pseudomonas aeruginosa, Bacillus subtilis, E. coli and Candida albicans.








Similar content being viewed by others
References
Abdi S, Nasiri M, Mesbahi A, Khani M (2017) Investigation of uranium (VI) adsorption by polypyrrole. J Hazard Mater 332:132–139
Abou-Zeid RE, Dacrory S, Ali KA, Kamel S (2018) Novel method of preparation of tricarboxylic cellulose nanofiber for efficient removal of heavy metal ions from aqueous solution. Int J Biol Macromol 119:207–214
Akl EM, Dacrory S, Abdel-Aziz MS, Kamel S & Fahim AM (2020) Preparation and characterization of novel antibacterial blended films based on modified carboxymethyl cellulose/phenolic compounds. Polym Bulle 1–25 (in press)
Choy CC, Korfiatis GP, Meng X (2006) Removal of depleted uranium from contaminated soils. J Hazard Mater 136:53–60
Dacrory S, Fahim AM (2019) Synthesis, anti-proliferative activity, Computational studies of tetrazole cellulose utilizing different homogenous catalyst. Carbohydr Polym 229:115537
Dacrory S, Abou-Yousef H, Abou-Zeid RE, Kamel S, Abdel-Aziz MS, Elbadry M (2018a) Preparation and characterization of eco-friendly carboxymethyl cellulose antimicrobial nanocomposite hydrogels. J Renew Mater 6:536–547
Dacrory S, Abou-Yousef H, Abouzeid RE, Kamel S, Abdel-aziz MS, El-badry M (2018b) Antimicrobial cellulosic hydrogel from olive oil industrial residue. Int J Biol Macromol 117:179–188
Dacrory S, Abou-Yousef H, Kamel S, Turky G (2019a) Development of biodegradable semiconducting foam based on micro-fibrillated cellulose/Cu-NPs. Int J Biol Macromol 132:351–359
Dacrory S, Abou-Yousef H, Kamel S, Abou-Zeid RE, Abdel-Aziz MS, Elbadry M (2019b) Functionalization and cross-linking of carboxymethyl cellulose in aqueous media. Cellul Chem Technol 53(1–2):23–33
Dacrory S, Moussa M, Turky G, Kamel S (2020) In situ synthesis of Fe3O4@ cyanoethyl cellulose composite as antimicrobial and semiconducting film. Carbohydr Polym 236:116032
Danko B, Dybczyński RS, Samczyński Z, Gajda D, Herdzik-Koniecko I, Zakrzewska-Kołtuniewicz G, Chajduk E, Kulisa K (2017) Ion exchange investigation for recovery of uranium from acidic pregnant leach solutions. Nukleonika 62:213–221
Deng B, Liu N, Wang H, Jiang S (2010) Progress in toxicity of uranium. J Chin J Radiat Health 19:113–116
Eliwa AA (2017) Kinetics and thermodynamics of carbonate dissolution process of uranium from Abu-Zeniema wet crude uranium concentrates. J Radioanal Nucl Chem 312:1–11
Favre-Réguillon A, Lebuzit G, Murat D, Foos J, Mansour C, Draye M (2008) Selective removal of dissolved uranium in drinking water by nanofiltration. Water Res 42:1160–1166
Freundlich H (1906) Over the adsorption in solution. J Phys Chem 57:1100–1107
Ghasemi Torkabad M, Keshtkar A, Safdari S (2017) Uranium membrane separation from binary aqueous solutions of UO22+ − K+ and UO22+ − Ca2+ by the nanofiltration process. Sep Sci Technol 52:1095–1105
Haggag ESA, Abdelsamad AA, Masoud AM (2019) Potentiality of uranium extraction from acidic leach liquor by polyacrylamide-acrylic acid titanium silicate composite adsorbent. Int J Environ Anal Chem 100(2):1–21
Ho Y-S, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Kamala KH, Dacroryb S, Alic SS, Alid KA, Kamelb S (2019) Adsorption of Fe ions by modified carrageenan beads with tricarboxy cellulose: kinetics study and four isotherm models. Desalin Water Treat 165:281–289
Khattab TA, Dacrory S, Abou-Yousef H, Kamel S (2019a) Development of microporous cellulose-based smart xerogel reversible sensor via freeze drying for naked-eye detection of ammonia gas. Carbohydr polym 210:196–203
Khattab TA, Dacrory S, Abou-Yousef H, Kamel S (2019b) Smart microfibrillated cellulose as swab sponge-like aerogel for real-time colorimetric naked-eye sweat monitoring. Talanta 205:120166
Kiegiel K, Abramowska A, Biełuszka P, Zakrzewska-Kołtuniewicz G, Wołkowicz S (2017) Solvent extraction of uranium from leach solutions obtained in processing of Polish low-grade ores. J Radioanal Nucl Chem 311:589–598
Lagergren S (1898) Zur theorie der sogenannten adsorption geloster stoffe. Kungliga svenska vetenskapsakademiens Handl 24:1–39
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Li X, Li F, Jin Y, Jiang C (2015) The uptake of uranium by tea wastes investigated by batch, spectroscopic and modeling techniques. J Mol Liq 209:413–418
Li D, Ye Y, Li D, Li X, Mu C (2016) Biological properties of dialdehyde carboxymethyl cellulose crosslinked gelatin–PEG composite hydrogel fibers for wound dressings. Carbohydr Polym 137:508–514
Liu Y, Cai Y, Xing N (2015) High-precision MC-ICP-MS determination method for U and Th long-lived nuclides in seawater. J Chin J Oceanogr 34:291–300
Mellah A, Chegrouche S, Barkat M (2006) The removal of uranium (VI) from aqueous solutions onto activated carbon: kinetic and thermodynamic investigations. J Colloid Interface Sci 296:434–441
Mu C, Guo J, Li X, Lin W, Li D (2012) Preparation and properties of dialdehyde carboxymethyl cellulose crosslinked gelatin edible films. Food Hydrocoll 27:22–29
Puigdomenech I (2006) HYDRA (Hydrochemical Equilibrium-Constant Database) and MEDUSA (Make Equilibrium Diagrams Using Sophisticated Algorithms) Programs. Royal Institute of Technology, Stockholm. Royal Institute of Technology, Sweden http://www.kemi.kth.se/medusa
Savvin S (1961) Analytical use of arsenazo III: determination of thorium, zirconium, uranium and rare earth elements. Talanta 8:673–685
Semião AJ, Rossiter HM, Schäfer AI (2010) Impact of organic matter and speciation on the behaviour of uranium in submerged ultrafiltration. J Membr Sci 348:174–180
Sprynskyy M, Kowalkowski T, Tutu H, Cukrowska EM, Buszewski B (2011) Adsorption performance of talc for uranium removal from aqueous solution. Chem Eng J 171:1185–1193
Tan Y (2017) Study on deep purification treatment of fluorine and uranium wastewater. Southwest University of Science and Technology
Villalobos-Rodríguez R, Montero-Cabrera M, Esparza-Ponce H, Herrera-Peraza E, Ballinas-Casarrubias M (2012) Uranium removal from water using cellulose triacetate membranes added with activated carbon. Appl Radiat Isot 70:872–881
Wang G, Liu J, Wang X, Xie Z, Deng N (2009) Adsorption of uranium (VI) from aqueous solution onto cross-linked chitosan. J Hazard Mater 168:1053–1058
Wang XL, Li Y, Huang J, Zhou YZ, Li BL, Liu DB (2019) Efficiency and mechanism of adsorption of low concentration uranium in water by extracellular polymeric substances. J Environ Radioact 197:81–89
Xiao-teng Z, Dong-mei J, Yi-qun X, Jun-chang C, Shuai H, Liang-shu X (2019) Adsorption of uranium (VI) from aqueous solution by modified rice stem. J Chem 2019:6409504
Zhu J, Liu Q, Li Z, Liu J, Zhang H, Li R, Wang J, Emelchenko G (2017) Recovery of uranium (VI) from aqueous solutions using a modified honeycomb-like porous carbon material. Dalton Trans 46:420–429
Acknowledgments
The authors would like to express their gratitude to the National Research Centre, Egypt and Egyptian Nuclear Materials Authority, for the financial support of the current work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
10570_2020_3272_MOESM1_ESM.docx
Supplementary data (including XRF-chart of radioactive deposits, Antimicrobial activity of cellulose p-toluidine against different microbes) associated with this article can be found, in the online version. (DOCX 243 kb)
Rights and permissions
About this article
Cite this article
Dacrory, S., Haggag, E.S.A., Masoud, A.M. et al. Innovative synthesis of modified cellulose derivative as a uranium adsorbent from carbonate solutions of radioactive deposits. Cellulose 27, 7093–7108 (2020). https://doi.org/10.1007/s10570-020-03272-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03272-w