Abstract
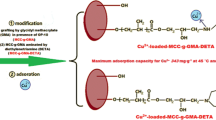
An environmental-friendly corn stalk cellulose-based adsorbent (DETA-g-GMA-MCC) was prepared by graft copolymerization and amination, whose adsorption properties of Ni2+ were fully studied. It was a spontaneously endothermic monolayer adsorption process and the rate-determining step was the chemisorption of Ni2+ on DETA-g-GMA-MCC, which was fitted well with pseudo-second-order kinetic model and Langmuir isotherm model. FTIR and XPS analysis demonstrated that the main functional groups of DETA-g-GMA-MCC for adsorption of Ni2+ were –NH2/–NH-groups, and Ni2+ was mainly adsorbed on the adsorbent surface by forming chelating bonds with two N atoms in –NH–/–NH2 groups. This relationship between the adsorbent microstructure and its adsorption behavior of Ni2+ was confirmed by comparison with the adsorption behavior of other metal ions on DETA-g-GMA-MCC and XPS analysis. Then the preparation of DETA-g-GMA-MCC was further improved according to the obtained adsorption mechanism. Zeta potential, FTIR and XPS results proved that more of amino groups were successfully introduced on the adsorbent surface after the improvement. The adsorbent with the highest content of amino groups (10.26 mmol/g) could be obtained when the initial pH value in the amination modification was 2.0, whose maximum adsorption capacity of Ni2+ was markedly increased from 188 (the original method) to 284 mg/g at 50 °C and pH 5.0. The improved adsorbent also showed a considerable adsorption capacity of Ni2+ after five cycles.
Graphic Abstract













Similar content being viewed by others
References
Anirudhan TS, Nima J, Divya PL (2013) Adsorption of chromium(VI) from aqueous solutions by glycidylmethacrylate-grafted-densified cellulose with quaternary ammonium groups. Appl Surf Sci 279:441–449
Anirudhan TS, Senan P (2011) Adsorption characteristics of cytochrome C onto cationic Langmuir monolayers of sulfonated poly(glycidylmethacrylate)-grafted cellulose: mass transfer analysis, isotherm modeling and thermodynamics. Chem Eng J 168:678–690
Bai L, Hu H, Fu W, Wan J, Cheng X, Zhuge L, Xiong L, Chen Q (2011) Synthesis of a novel silica-supported dithiocarbamate adsorbent and its properties for the removal of heavy metal ions. J Hazard Mater 195:261–275
Bartczak P, Norman M, Klapiszewski Ł, Karwańska N, Kawalec M, Baczyńska M, Wysokowski M, Zdarta J, Ciesielczyk F, Jesionowski T (2018) Removal of nickel(II) and lead(II) ions from aqueous solution using peat as a low-cost adsorbent: a kinetic and equilibrium study. Arabian J Chem 11:1209–1222
Chen CY (2003) Stability constants of water-soluble and latex types of chelating polymers containing iminodiacetic acid with some transition metal ions. Eur Polym J 39:991–1000
Chen CY (2005) Formation of silver nanoparticles on a chelating copolymer film containing iminodiacetic acid. Thin Solid Films 484:68–72
Coelho TC, Laus R, Mangrich AS, de Fávere VT, Laranjeira MCM (2007) Effect of heparin coating on epichlorohydrin cross-linked chitosan microspheres on the adsorption of copper (ii) ions. React Funct Polym 67:468–475
Conner GR (1978) Combination analysis of metal oxides using ESCA, AES, and SIMS. J Vac Sci Technol 15(2):343–347
Cui L, Wang Y, Gao L, Hu L, Yan L, Wei Q, Du B (2015) EDTA functionalized magnetic graphene oxide for removal of Pb(II), Hg(II) and Cu(II) in water treatment: adsorption mechanism and separation property. Chem Eng J 281:1–10
Dong C, Yang B, Xing Y, Xu H, Xiao J, Lin Q, Joseph EN (2016) Improving the colloidal stability of Cellulose nano-crystals by surface chemical grafting with polyacrylic acid. J Bioresour Bioprod 1(3):114–119
Duru Í, Ege D, Kamali AR (2016) Graphene oxides for removal of heavy and precious metals from wastewater. J Mater Sci 51:6097–6116
El-Khouly AS, Takahashi Y, Saafan AA, Kenawy E, Hafiz YA (2011) Study of heavy metal ion absorbance by amidoxime group introduced to cellulose-graft-polyacrylonitrile. J Appl Polym Sci 120:866–873
Fu FL, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manag 92:407–418
Gao H, Sun Y, Zhou J, Xu R, Duan H (2013) Mussel-inspired synthesis of polydopamine-functionalized graphene hydrogel as reusable adsorbents for water purification. ACS Appl Mat Interfaces 5:425–432
Gurung M, Adhikari BB, Gao XP, Alam S, Inoue K (2014) Sustainability in the metallurgical industry: chemically modified cellulose for selective biosorption of gold from mixtures of base metals in chloride media. Ind Eng Chem Res 53:8565–8576
Hajeeth T, Vijayalakshmi K, Gomathi T, Sudha PN (2013) Removal of Cu(II) and Ni(II) using cellulose extracted from sisal fiber and cellulose-g-acrylic acid copolymer. Int J Biolmacromol 62:59–65 (Complete)
He Z, Wang Y, Zhao T, Ye Z, Huang H (2014) Ultrasonication-assisted rapid determination of epoxide values in polymer mixtures containing epoxy resin. Anal Methods 6(12):4257–4261
Hossain MA, Ngo HH, Guo WS, Nghiem LD, Hai FI, Vigneswaran S, Nguyen TV (2014) Competitive adsorption of metals on cabbage waste from multi-metal solutions. Bioresour Technol 160:79–88
Jia Q, Li D, Gao X, Yan J, Ma Q, Meng F (2016) Hydrazinolyzed cellulose-g-polymethyl acrylate as adsorbent for efficient removal of Cu(II) and Ni(II) ions from aqueous solution. J Chem Technol Biot 91:1378–1386
Jing X, Liu F, Yang X, Ling P, Li L, Long C, Li A (2009) Adsorption performances and mechanisms of the newly synthesized N, N'-di (carboxymethyl) dithiocarbamate chelating resin toward divalent heavy metal ions from aqueous media. J Hazard Mater 167:589–596
Langmuir I (1918) The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Leinonen H, Lehto J (2000) Ion-exchange of nickel by iminodiacetic acid chelating resin Chelex 100. React Funct Polym 43:1–6
Lin S, Lai S, Leu H (2000) Removal of heavy metals from aqueous solution by chelating resin in a multistage adsorption process. J Hazard Mater 76:139–153
Liu C, Bai R, Ly QS (2008) Selective removal of copper and lead ions by diethylenetriamine-functionalized adsorbent: Behaviors and mechanisms. Water Res 42:1511–1522
Liu L, Xie J, Li Y, Zhang Q, Yao J (2016) Three-dimensional macroporous cellulose-based bioadsorbents for efficient removal of nickel ions from aqueous solution. Cellulose 23:723–736
Luo T, Tian X, Yang C, Luo W, Nie Y, Wang Y (2017) Polyethylenimine-Functionalized Corn Bract, an Agricultural Waste Material, for Efficient Removal and Recovery of Cr (VI) from Aqueous Solution. J Agric Food Chem 65:7153–7158
Luo Z, Murray BS, Ross AL, Povey MJW, Morgan MRA, Day AJ (2012) Effects of pH on the ability of flavonoids to act as Pickering emulsion stabilizers. Colloid Surf B 92:84–90
Ma A, Abushaikha A, Allen SJ, McKay G (2019) Ion exchange homogeneous surface diffusion modelling by binary site resin for the removal of nickel ions from wastewater in fixed beds. Chem Eng J 358:1–10
Maatar W, Boufi S (2015) Poly(methacylic acid-co-maleic acid) grafted nanofibrillated cellulose as a reusable novel heavy metal ions adsorbent. Carbohydr Polym 126:199–207
Mashhadzadeh AH, Fathalian M, Ahangari MG, Shahavi MH (2018) DFT study of Ni, Cu, Cd and Ag heavy metal atom adsorption onto the surface of the zinc-oxide nanotube and zinc-oxide graphene-like structure. Mater Chem Phys 220:366–373
Mohamed MF, Essawy HA, Ammar NS, Ibrahim HS (2017) Potassium fulvate-modified graft copolymer of acrylic acid onto cellulose as efficient chelating polymeric sorbent. Int J Biol Macromol 94:771–780
Nguyen TAH, Ngo HH, Guo WS, Zhang J, Liang S, Yue QY, Li Q, Nguyen TV (2013) Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour Technol 148:574–585
O’Connell DW, Birkinshaw C, O’Dwyer TF (2008) Heavy metal adsorbents prepared from the modification of cellulose: a review. Bioresour Technol 99:6709–6724
Orozco-Guareño E, Santiago-Gutiérrez FS, Morán-Quiroz JL, Hernandez-Olmos SL, Soto V, de la Cruz W, ManrÍquez R, Gomez-Salazar S (2010) Removal of Cu(II) ions from aqueous streams using poly(acrylic acid-co-acrylamide) hydrogels. J Colloid Interface Sci 349:583–593
Pederson LR (1982) Two-dimensional chemical-state plot for lead using XPS. J Electron Spectrosc Relat Phenom 28(2):203–209
Pehlivan E, Altun T (2006) The study of various parameters affecting the ion exchange of Cu2+, Zn2+, Ni2+, Cd2+ and Pb2+ from aqueous solution on Doewex 50W synthetic resin. J Hazard Mater 134:149–156
Qiu X, Hu S (2013) “Smart” materials based on cellulose: a review of the preparations, properties, and applications. Materials 6:738–781
Reischl M, Stana-Kleinschek K, Ribitsch V (2006) Electrokinetic investigations of oriented cellulose polymers. Macromol Symp 244(1):31–47
Rodriguez JA, Jirsak T, Dvorak J, Sambasivan S, Fischer D (2000) Reaction of NO2 with Zn and ZnO: photoemission, XANES, and density functional studies on the formation of NO3. J Phys Chem B 104:319–328
Salgin S, Salgin U, Bahadir S (2012) Zeta potentials and isoelectric points of biomolecules: the effects of ion types and ionic strengths. Int J Electrochem Sci 7(12):12404–12414
SolomunT SA, Sturm H, Illenberger E (2004) Reactions of amide group with fluorine as revealed with surface analytics. Chem Phys Lett 387:312–316
Thakur AK, Nisola GM, Limjuco LA, Parohinog KJ (2017) Polyethylenimine-modified mesoporous silica adsorbent for simultaneous removal of Cd(II) and Ni(II) from aqueous solution. J Ind Eng Chem 49:133–144
Wang Q, Zheng C, Shen Z, Lu Q, He C, Zhang T, Liu J (2019) Polyethyleneimine and carbon disulfide co-modified alkaline lignin for removal of Pb2+ ions from water. Chem Eng J 359:265–274
Wehner PS, Mercer PN, Apai G (1983) Interaction of H2 and CO with Rh4 (CO)12 supported on ZnO. J Catal 84(1):244–247
WenSSY RML, Arshad SE, Surugau NL, Musta B (2012) Synthesis and characterization of poly(hydroxamic acid)-poly(amidoxime) chelating ligands from polymer-grafted acacia cellulose. J Appl Polym Sci 124:4443–4451
Wu Y, Jiang Y, Li Y, Wang R (2019) Optimum synthesis of an amino functionalized microcrystalline cellulose from corn stalk for removal of aqueous Cu2+. Cellulose 26:805–821
Zhen H, Xu Q, Hu Y, Cheng J (2012) Characteristics of heavy metals capturing agent dithiocarbamate (DTC) for treatment of ethylene diamine tetraacetic acid–Cu (EDTA–Cu) contaminated wastewater. Chem Eng J 209:547–557
Zhu T, Lu L (2004) X-ray diffraction and photoelectron spectroscopic studies of (001)-oriented Pb(Zr0.52Ti0.48)O-3 thin films prepared by laser ablation. J Appl Phys 95:241–247
Acknowledgments
The authors gratefully acknowledge the financial support provided by National Natural Science Foundations of China (No. 21666021, 21968018 and 21706112) and National Natural Science Foundation of Jiangxi Province (No. 20161BAB203076).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors all declare no conflict of interest in connection with the work submitted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, Y., Jiang, Y., Deng, Q. et al. Insights into performance, structure-property relationship and adsorption mechanism to efficiently remove Ni2+ by corn stalk cellulose functionalized with amino groups. Cellulose 27, 5149–5168 (2020). https://doi.org/10.1007/s10570-020-03098-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-020-03098-6