Abstract
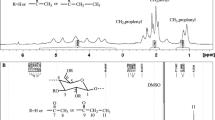
The transesterification reaction between cellulose and vinyl esters is regarded as a clean and facile strategy for the tunable synthesis of cellulose esters. In this study, a series of cellulose esters with degrees of substitution from 0.58 to 3.0 have been prepared successfully under mild conditions without adding any external catalysts when cellulose was dissolved in the 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU)/DMSO/CO2 solvent system and then followed by adding equimolar amounts of long chain fatty, aromatic, branched and steric vinyl esters. The optimization study of different reaction parameters like reaction time, temperature and amounts of substrates demonstrates that the reaction can proceed smoothly even at room temperature. This is evidenced by a cellulose benzoate with a DS of 2.6 obtained when the reaction is performed at 25 °C in 4 h. The as-prepared cellulose esters structure has been confirmed by 1H NMR, 13C NMR and FTIR, and material thermal properties were evaluated by DSC and TGA for an in-depth understanding the relationship between the cellulose esters structure and properties. It is believed that the DBU not only acts as a reagent for the CO2-derivative dissolution of cellulose in DMSO, but also acts as an in situ organocatalyst for the subsequent transesterification reaction.
Graphical abstract
Cellulose esters have been prepared successfully at low temperature using long chain fatty acids, aromatic, branched and steric vinyl esters as acyl donors in the DMSO/DBU/CO2 solvents system, and the DBU not only acts as reagents for the CO2-derivative dissolution of cellulose in DMSO, acts as an organocatalyst for the subsequent derivatisation.







Similar content being viewed by others
References
Barthel S, Heinze T (2006) Acylation and carbanilation of cellulose in ionic liquids. Green Chem 8:301–306
Cao Y, Zhang J, He J, Li H, Zhang Y (2010) Homogeneous acetylation of cellulose at relatively high concentrations in an ionic liquid. Chin J Chem Eng 18:515–522
Cao X, Sun S, Peng X, Zhong L, Sun R, Jiang D (2013) Rapid synthesis of cellulose esters by transesterification of cellulose with vinyl esters under the catalysis of NaOH or KOH in DMSO. J Agric Food Chem 61:2489–2495
Cao X, Peng X, Zhong L, Sun S, Yang D, Zhang X, Sun R (2014) A novel transesterification system to rapidly synthesize cellulose aliphatic esters. Cellulose 21:581–594
Çetin NS, Tingaut P, Özmen N, Henry N, Harper D, Dadmun M, Sèbe G (2009) Acetylation of cellulose nanowhiskers with vinyl acetate under moderate conditions. Macromol Biosci 9:997–1003
Chen M-J, Li R-M, Zhang X-Q, Feng J, Feng J, Liu C-F, Shi Q-S (2016) Homogeneous transesterification of sugarcane bagasse toward sustainable plastics. ACS Sustain Chem Eng 5:360–366
Cumpstey I (2013) Chemical modification of polysaccharides. ISRN Org Chem 2013:27
Dicke R (2004) A straight way to regioselectively functionalized polysaccharide esters. Cellulose 11:255–263
Freire CSR, Silvestre AJD, Neto CP, Belgacem MN, Gandini A (2006) Controlled heterogeneous modification of cellulose fibers with fatty acids: effect of reaction conditions on the extent of esterification and fiber properties. J Appl Polym Sci 100:1093–1102
Gao K, Chen Q, Hu Y, Zheng Q, Xie H (2016) Preparation and characterization of cellulose benzoate in CO2/DBU/DMSO. Sci Sin Chim 46:1337–1342
Gericke M, Fardim P, Heinze T (2012) Ionic liquids-promising but challenging solvents for homogeneous derivatization of cellulose. Molecules 17:7458–7502
Hanabusa H, Izgorodina EI, Suzuki S, Takeoka Y, Rikukawa M, Yoshizawa-Fujita M (2018) Cellulose-dissolving protic ionic liquids as low cost catalysts for direct transesterification reactions of cellulose. Green Chem 20:1412–1422
Heinze T, Dicke R, Koschella A, Kull AH, Klohr EA, Koch W (2000) Effective preparation of cellulose derivatives in a new simple cellulose solvent. Macromol Chem Phys 201:627–631
Hinner LP, Wissner JL, Beurer A, Nebel BA, Hauer B (2016) Homogeneous vinyl ester-based synthesis of different cellulose derivatives in 1-ethyl-3-methyl-imidazolium acetate. Green Chem 18:6099–6107
Itoh T, Nishimura Y, Ouchi N, Hayase S (2003) 1-Butyl-2,3-dimethylimidazolium tetrafluoroborate: the most desirable ionic liquid solvent for recycling use of enzyme in lipase-catalyzed transesterification using vinyl acetate as acyl donor. J Mol Catal B Enzym 26:41–45
Junistia L, Sugih AK, Manurung R, Picchioni F, Janssen LPBM, Heeres HJ (2008) Synthesis of higher fatty acid starch esters using vinyl laurate and stearate as reactants. Starch-Stärke 60:667–675
Klemm D, Heublein B, Fink H-P, Bohn A (2005) Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Inter Ed 44:3358–3393
Mormann W, Al-Higari M (2004) Acylation of starch with vinyl acetate in water. Starch-Starke 56:118–121
Schenzel A, Hufendiek A, Barner-Kowollik C, Meier MAR (2014) Catalytic transesterification of cellulose in ionic liquids: sustainable access to cellulose esters. Green Chem 16:3266–3271
Shogren RL, Biswas A (2010) Acetylation of starch with vinyl acetate in imidazolium ionic liquids and characterization of acetate distribution. Carbohydr Polym 81:149–151
Song Y, Sun Y, Zhang X, Zhou J, Zhang L (2008) Homogeneous quaternization of cellulose in NaOH/urea aqueous solutions as gene carriers. Biomacromol 9:2259–2264
Song L, Yang Y, Xie H, Liu E (2015) Cellulose dissolution and in situ grafting in a reversible system using an organocatalyst and carbon dioxide. ChemSusChem 8:3217–3221
Tsuboi M (1957) Infrared spectrum and crystal structure of cellulose. J Polym Sci 25:159–171
Vicente G, Martinez M, Aracil J (2004) Integrated biodiesel production: a comparison of different homogeneous catalysts systems. Bioresour Technol 92:297–305
Xiao P, Zhang J, Feng Y, Wu J, He J, Zhang J (2014) Synthesis, characterization and properties of novel cellulose derivatives containing phosphorus: cellulose diphenyl phosphate and its mixed esters. Cellulose 21:2369–2378
Xie J, Hsieh YL (2001) Enzyme-catalyzed transesterification of vinyl esters on cellulose solids. J Polym Sci Part A Polym Chem 39:1931–1939
Xu Q, Song L, Zhang L, Hu G, Du J, Liu E, Zheng Q, Liu Y, Li N, Xie H (2017) Organocatalytic cellulose dissolution and in situ grafting of epsilon-caprolactone via ROP in a reversible DBU/DMSO/CO2 system. Chemistryselect 2:7128–7134
Xu Q, Song L, Zhang L, Hu G, Chen Q, Liu E, Liu Y, Zheng Q, Xie H, Li N (2018) Synthesis of cellulose acetate propionate and cellulose acetate butyrate in a CO2/DBU/DMSO system. Cellulose 25:205–216
Yang Y, Xie H, Liu E (2014) Acylation of cellulose in reversible ionic liquids. Green Chem 16:3018–3023
Yang Y, Song L, Peng C, Liu E, Xie H (2015) Activating cellulose via its reversible reaction with CO2 in the presence of 1,8-diazabicyclo 5.4.0 -undec-7-ene for the efficient synthesis of cellulose acetate. Green Chem 17:2758–2763
Zhang J, Wu J, Cao Y, Sang S, Zhang J, He J (2009) Synthesis of cellulose benzoates under homogeneous conditions in an ionic liquid. Cellulose 16:299–308
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (Grant No. 31270637 and 21574030); Science and Technology Department of Guizhou Province (Grant No. Natural Science Key Fund [2016]1402), (Grant No. Platform & Talents [2016]5652); Open research fund of Key Laboratory of Pulp and Paper Science & Technology of Ministry of Education of China (08031339); Foundation of State Key Laboratory of Coal Conversion (Grant No. J17-18-907); Excellent Scientific Innovative Talent Programme from Education Department of Guizhou Province (Grant No. KY[2015]479).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There are no conflicts to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, H., Yang, F., Du, J. et al. Efficient transesterification reaction of cellulose with vinyl esters in DBU/DMSO/CO2 solvent system at low temperature. Cellulose 25, 6935–6945 (2018). https://doi.org/10.1007/s10570-018-2078-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-2078-7