Abstract
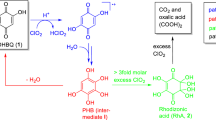
5,8-Dihydroxy-[1,4]-naphthoquinone (DHNQ) is one of the key chromophores occurring in all types of aged cellulosics. This study investigates the degradation of DHNQ by chlorine dioxide at moderately acidic (pH 3) conditions, corresponding to the conditions of industrial bleaching (“D stage”). The degradation involves three major pathways. As initial reaction, a hydrogen transfer from DHNQ to chlorine dioxide via a PCET mechanism occurs to form a radical DHNQ· and chlorous acid. DHNQ· is then attacked by water to give a pentahydroxynaphthalene radical PHN· that is stabilized by strong delocalization of the non-paired electron into its aromatic ring. PHN· immediately disproportionates to give the observable intermediate 1,2,4,5,8-pentahydroxynapththalene (I), which was comprehensively confirmed by NMR and MS (path A). In the presence of excess ClO2, I is immediately further oxidized into acetic acid, glycolic acid, oxalic acid and CO2 as the final, stable, and non-colored products (path C). In the absence of excess ClO2, elimination of water from I regenerates DHNQ (path B), so that at roughly equimolar DHNQ/ClO2 ratios ClO2 is fully consumed while a major part of DHNQ is recovered. To avoid such DHNQ “recycling” under ClO2 consumption—and to completely degrade DHNQ to colorless degradation products instead—ClO2 must be applied in at least fivefold molar excess relative to DHNQ.
Graphical Abstract









Similar content being viewed by others
References
Aguilar CAH, Narayanan J, Singh N, Thangarasu P (2014) Kinetics and mechanism for. the oxidation of anilines by ClO2: a combined experimental and computational study. J Phys Org Chem 27:440–449
Arnone A, Merlini L, Nasini G, Vajna de Pava O (2007) Asymmetric Diels–Alder reactions. Part 6. Regio- and stereo-selective cycloadditions of 5-(2′,3′,4′,6′-tetra-O-acetyl-β-D-glucopyranosyloxy)-1,4-naphthoquinone. Synth Commun 37:2569–2573
Betts RL, Murphy ST, Johnson CR (2004) Enzymatic desymmetrization/resolution of epoxydiols derived from 1,4-naphthoquinone, 5-hydroxy-1,4-naphthoquinone and 5,8-dihydroxy-1,4- naphthoquinone. Tetrahedron Asymmetry 15:2853–2860
Cuellar MA, Salas C, Cortes MJ, Morello A, Maya JD, Preite MD (2003) Synthesis and in vitro trypanocide activity of several polycyclic drimane–quinone derivatives. Bioorg Med Chem 11:2489–2497
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao, O, Nakai H, Vreven T, Montgomery Jr. JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd J, Brothers EN, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian Inc., Wallingford
Greco G, Panzella L, Pezzella A, Napolitano A, d’Ischia M (2010) Reaction of dihydrolipoic acid with juglone and related naphthoquinones: unmasking of a spirocyclic 1,3-dithiane intermediate en route to naphtho[1,4]dithiepines. Tetrahedron 66:3912–3916
Hosoya T, French AD, Rosenau T (2013) Chemistry of 5,8-dihydroxy-[1,4]-naphthoquinone, a key chromophore in aged cellulosics. Mini Rev Org Chem 10(3):309–315
Hull LA, Davis GT, Rosenblatt DH, Williams HKR, Weglein RC (1967) Oxidations of amines. III. Duality of mechanism in the reaction of amines with chlorine dioxide. J Am Chem Soc 89:1163–1170
Kelly TR, Fu Y, Sieglen JT Jr., De Silva H (2000) Synthesis of an orange anthrathiophene pigment isolated from a Japanese bryozoan. Org Lett 2:2351–2352
Korntner P, Hosoya T, Dietz T, Eibinger K, Reiter H, Spitzbart M, Röder T, Borgards A, Kreiner W, Mahler AK, Winter H, French AD, Henniges U, Potthast A, Rosenau T (2015) Chromophores in lignin-free cellulosic materials belong to three compound classes. Chromophores in cellulosics, XII. Cellulose 22(2):1053–1062
Lanari D, Marrocchi A, Minuti L, Taticchi A, Gacs-Baitz E (2002) Synthesis of some new enantiopure [2.2]paracyclophanes bearing polycyclic aromatic subunits. Tetrahedron Asymmetry 13:1331–1335
Lehtimaa T, Kuitunen S, Tarvo V, Vuorinen T (2010) Reactions of aldehydes with chlorous acid and chlorine in dioxide bleaching. Holzforschung 64:555–561
Leigh JK, Rajput J, Richardson DE (2014) Kinetics and mechanism of styrene epoxidation by chlorite: role of chlorine dioxide. Inorg Chem 53:6715–6727
Liftinger E, Zweckmair T, Schild G, Eilenberger G, Böhmdorfer S, Rosenau T, Potthast A (2015) Analysis of degradation products in rayon spinning baths. Holzforschung 69(6):695–702
Mital A, Negi VS, Ramachandran U (2008) Synthesis and biological evaluation of naphthalene-1,4-dione derivatives as potent antimycobacterial agents. Med Chem 4:492–497
Napolitano MJ, Green BJ, Nicoson JS, Margerum DW (2005) Chlorine dioxide oxidations of tyrosine, N-acetyltyrosine, and dopa. Chem Res Toxicol 18:501–508
Potthast A, Schedl A, Zweckmair T, Kikul F, Bacher M, Rosenau T (2018) Pushing the limits: quantification of chromophores in real-world paper samples by GC-ECD and EI-GC-MS. Talanta 179:693–699
Rosenau T, Potthast A, Milacher W, Hofinger A, Kosma P (2004) Isolation and identification of residual chromophores in cellulosic materials. Polymer 45(19):6437–6443
Rosenau T, Potthast A, Kosma P, Suess H-U, Nimmerfroh N (2007) First isolation and identification of residual chromophores from aged bleached pulp samples. Holzforschung 61(6):656–661
Rosenau T, Potthast A, Kosma P, Suess HU, Nimmerfroh N (2008) Chromophores in aged hardwood pulp—their structure and degradation potential. TAPPI J 1:24–30
Schedl A, Korntner P, Zweckmair T, Rosenau T, Potthast A (2016) Detection of cellulose-derived chromophores by ambient ionization-MS. Anal Chem 88:1253–1258
Schedl A, Zweckmair T, Kikul F, Henniges U, Rosenau T, Potthast A (2017) Aging of paper—ultra-fast quantification of 2,5-dihydroxyacetophenone, as a key chromophore in cellulosics, by reactive paper spray-mass spectrometry. Talanta 167:672–680
Tandon VK, Maurya HK (2009) Naphtho [2, 3-b][1, 4]-thiazine-5, 10-diones and 3-substituted-1, 4-dioxo-1, 4-dihydronaphthalen-2-yl-thioalkanoate derivatives: synthesis and biological evaluation. Tetrahedron Lett 50:5896–5902
Tandon VK, Singh RV, Yadav DB (2004) Synthesis and evaluation of novel 1,4-naphthoquinone derivatives as antiviral, antifungal and anticancer agents. Bioorg MedChem Lett 14:2901–2904
Tandon VK, Yadav DB, Chaturvedi AK, Shukla PK (2005a) Synthesis of (1,4)-naphthoquinono-[3,2-c]-1H-pyrazoles and their (1,4)-naphthohydroquinone derivatives as antifungal, antibacterial, and anticancer agents. Bioorg Med Chem Lett 15:3288–3291
Tandon VK, Yadav DB, Singh RV, Chaturvedi AK, Shukla PK (2005b) Synthesis and biological evaluation of novel (L)-alpha-amino acid methyl ester, heteroalkyl, and aryl substituted 1,4-naphthoquinone derivatives as antifungal and antibacterial agents. Bioorg Med Chem Lett 15:5324–5328
Tandon VK, Maurya HK, Verma MK, Kumar R, Shukla PK (2010) ‘On water’ assisted synthesis and biological evaluation of nitrogen and sulfur containing hetero-1,4-naphthoquinones as potent antifungal and antibacterial agents. Eur J Med Chem 45:2418–2426
Tishchenko O, Truhlar DG, Ceulemans A, Nguyen MT (2008) A unified perspective on the hydrogen atom transfer and proton-coupled electron transfer mechanisms in terms of topographic features of the ground and excited potential energy surfaces as exemplified by the reaction between phenol and radicals. J Am Chem Soc 130:7000–7010
Valderrama JA, Benites J, Cortes M, Pessoa-Mahana H, Prina E, Fournet A (2003) Studies on quinones: part 38. Synthesis and leishmanicidal activity of sesquiterpene 1,4-quinones. Bioorg Med Chem 11:4713–4718
Wenger J, Schedl A, Zweckmair T, Schuhmacher R, Rechthaler J, Herbinger B, Rosenau T, Potthast A (2015) Challenges to detect key-chromophores by paperspray mass spectrometry on cellulosic material. In: Conference proceedings, 18th ISWFPC international symposium on wood, fibre and pulping chemistry, Vienna, Austria, September 09–11, 2015, Vol. II, P121; ISBN: 978-3-900932-24-4
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Acc 120(1–3):215–241
Zhou J, Duan L, Chen H, Ren X, Zhang Z, Zhou F, Liu J, Pei D, Ding K (2009) Atovaquone derivatives as potent cytotoxic and apoptosis inducing agents. Bioorg Med Chem Lett 19:5091–5094
Zwirchmayr NS, Hosoya T, Henniges U, Gille L, Bacher M, Furtmüller P, Rosenau T (2017) Degradation of the cellulosic key chromophore 5,8-dihydroxy-[1,4]-naphthoquinone by hydrogen peroxide under alkaline conditions. J Org Chem 82(21):11558–11565
Acknowledgments
We performed quantum chemical calculations with the workstation in the Sakaki group, Fukui institute for fundamental chemistry at Kyoto University, Japan, and would like to thank for the access. The financial support of the Austrian Christian Doppler Research Society (CDG) through the CD-lab “Advanced cellulose chemistry and analytics” and the Austrian Research promotion Agency (FFG), project “Chromophores-II”, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosoya, T., Zwirchmayr, N.S., Klinger, K.M. et al. Chromophores in cellulosics, XVIII. Degradation of the cellulosic key chromophore 5,8-dihydroxy-[1,4]-naphthoquinone under conditions of chlorine dioxide pulp bleaching: a combined experimental and theoretical study. Cellulose 25, 4941–4954 (2018). https://doi.org/10.1007/s10570-018-1912-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-018-1912-2