Abstract
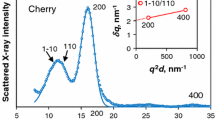
Cellulose crystals in fibrovascular bundles of sugarcane culms were throughly characterized by X-ray diffraction, with area-detector patterns acquired in fiber geometry. It was observed that microfibril angles are, on average, higher for bundles from pith compared to bundles from intermediate regions and rind, and higher for ratoon canes compared to plant canes. On the other hand, microfibril angles do not differ significantly among sugarcane cultivars, internode positions in the culm, or ratoon cane cut cycles. Broadening analyses of diffraction peaks yield crystal sizes (crystal width from equatorial 200 reflection and apparent crystal lengths from meridional 002 and 004 reflections) similar to other plant species (woods and bamboo). In addition, compared to reference cellulose Iβ, the 200, 110, and 110 diffraction peaks from sugarcane cellulose are notably shifted. These shifts indicate pronounced crystallite distortions, with expanded intersheet spacing d 200 , contracted d 1 1 0 /d 110 , and monoclinic angle γ closer to 90°. Our findings deepen the understanding of the fine structure and variability of sugarcane lignocellulose.







Similar content being viewed by others
References
Abe K, Yamamoto H (2005) Mechanical interaction between cellulose microfibril and matrix substance in wood cell wall determined by X-ray diffraction. J Wood Sci 51:334–338
Andersson S, Serimaa R, Paakkari T, Saranpää P, Pesonen E (2003) Crystallinity of wood and the size of cellulose crystallites in Norway spruce (Picea abies). J Wood Sci 49:531–537
Barnett JR, Bonham VA (2004) Cellulose microfibril angle in the cell wall of wood fibres. Biol Rev 79:461–472
Buckeridge MS, de Souza AP, Arundale RA, Anderson-Teixeira KJ, DeLucia E (2011) Ethanol from sugarcane in Brazil: a ‘midway’ strategy for increasing ethanol production while maximizing environmental benefits. GCB Bioenergy 4:119–126
Burgert I, Fratzl P (2009) Plants control the properties and actuation of their organs through the orientation of cellulose fibrils in their cell walls. Integr Comp Biol 49:69–79
Cave ID (1997) Theory of X-ray measurement of microfibril angle in wood. Wood Sci Tech 31:225–234
Cortez LAB (ed) (2010) Sugarcane bioethanol—R&D for productivity and sustainability. Blucher, São Paulo
Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6:850–861
Delhez R, de Keijser TH, Langford JI, Louër D, Mittemeijer EJ, Sonneveld EJ (1993) Crystal imperfection broadening and peak shape in the Rietveld method. In: Young RA (ed) The Rietveld method. Oxford University Press, New York, pp 132–166
dos Santos WD, Gómez EO, Buckeridge MS (2011) Bioenergy and the sustainable revolution. In: Goldman GH, Buckeridge MS (eds) Routes to cellulosic ethanol. Springer, New York, pp 15–26
Driemeier C, Calligaris GA (2011) Theoretical and experimental developments for accurate determination of crystallinity of cellulose I materials. J Appl Crystallogr 44:184–192
Driemeier C, Pimenta MTB, Rocha GJM, Oliveira MM, Mello DB, Maziero P, Gonçalves AR (2011) Evolution of cellulose crystals during prehydrolysis and soda delignification of sugarcane lignocellulose. Cellulose 18:1509–1519
Emons AMC, Mulder BM (2000) How the deposition of cellulose microfibrils builds cell wall architecture. Trends Plant Sci 5:35–40
Esau K (1977) Anatomy of seed plants. Wiley, New York
Fernandes AN, Thomas LH, Altaner CM, Callow P, Forsyth VT, Apperley DC, Kennedy CJ, Jarvis MC (2011) Nanostructure of cellulose microfibrils in spruce wood. Proc Natl Acad Sci USA 108:E1195–E1203
Ferrari M, Lutterotti L (1994) Method for the simultaneous determination of anisotropic residual-stresses and texture by X-ray diffraction. J Appl Phys 76:7246–7255
Goldemberg J, Guardabassi P (2010) The potential for first-generation ethanol production from sugarcane. Biofuels Bioprod Bioref 4:17–24
Hosemann R, Hindeleh AM (1995) Structure of crystalline and paracrystalline condensed matter. J Macromol Sci B Phys 34:327–356
Ioelovich M (2008) Cellulose as a nanostructured polymer: a short review. BioResources 3:1403–1418
Jakob HF, Fengel D, Tschegg SE, Fratzl P (1995) The elementary cellulose fibril in Picea abies: comparison of transmission electron microscopy, small-angle X-ray scattering, and wide-angle X-ray scattering results. Macromolecules 28:8782–8787
Keckes J, Burgertt I, Frühmann K, Müller M, Kölln K, Hamilton M, Burghammer M, Roth SV, Stanzl-Tschegg S, Fratzl P (2003) Cell-wall recovery after irreversible deformation of wood. Nat Mat 2:810–814
Kulshreshtha AK, Patil NB, Dweltz NE, Radhakrishnan T (1969) Axial order in ramie. Text Res J 39:1158–1161
Lutterotti L, Matthies S, Wenk H-R, Schultz AJ, Richardson J (1997) Combined texture and structure analysis of deformed limestone from time-of-flight neutron diffraction spectra. J Appl Phys 81:594–600
Nishiyama Y, Langan P, Chanzy H (2002) Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J Am Chem Soc 124:9074–9082
Okano T, Koyanagi A (1986) Structural variation of native cellulose related to its source. Biopolymers 25:851–861
Parameswaran N, Liese W (1976) On the fine structure of bamboo fibres. Wood Sci Tech 10:231–246
Prata AP, Menezes NL, Mazzoni-Viveiros SC, Wanderley MGL, Thomas WW (2007) Anatomia do escapo e rizoma de espécies brasileiras de Bulbostylis Kunth (Cyperacea). Rev Bras Botânica 30:245–256
Siqueira G, Milagres AMF, Carvalho W, Koch G, Ferraz A (2011) Topochemical distribution of lignin and hydroxycinnamic acids in sugar-cane cell walls and its correlation with the enzymatic hydrolysis of polysaccharides. Biotechnol Biofuels 4:7
Skaar C (1988) Wood-water relations. Springer, Berlin
Soccol CR, Vandenberghe LPD, Medeiros ABP, Karp SG, Buckeridge M, Ramos LP, Pitarelo AP, Ferreira-Leitao V, Gottschalk LMF, Ferrara MA, Bom EPD, de Moraes LMP, Araujo JD (2010) Bioethanol from lignocelluloses: status and perspectives in Brazil. Bioresour Technol 101:4820–4825
Somerville C, Youngs H, Taylor C, Davis SC, Long SP (2010) Feedstocks for lignocellulosic biofuels. Science 329:790–792
Thygesen A, Oddershede J, Lilholt H, Thomsen AB, Ståhl K (2005) On the determination of crystallinity and cellulose content in plant fibres. Cellulose 12:563–576
Triana O, Leonard M, Saavedra F, Fernández N, Gálvez G, Peña E (1990) Atlas del bagazo de la caña de azúcar, 1st edn. GEPLACEA, México
Van Dillewijn C (1951) Botánica de canã de azúcar. Edicion Revolucionaria, Instituto del Libro, La Habana
Wada M, Heux L, Sugiyama J (2004) Polymorphism of cellulose I family: reinvestigation of cellulose IVI. Biomacromolecules 5:1385–1391
Wang Y, Leppänen K, Andersson S et al (2012) Studies on the nanostructure of the cell wall of bamboo using X-ray scattering. Wood Sci Techn 46:317–332
Warren BE (1990) X-ray diffraction. Dover Publications, New York
Zabler S, Paris O, Burgert I, Fratzl P (2010) Moisture changes in the plant cell wall force cellulose crystallites to deform. J Struc Biol 171:133–141
Acknowledgments
Authors thank family Grandis from Fazenda Vitória for kind provision of the sugarcane culms. Research supported by LNLS – Brazilian Synchrotron Light Laboratory and LNBio – Brazilian Biosciences National Laboratory (project GAR6293) and by FAPESP (project 2010/05523-3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Driemeier, C., Santos, W.D. & Buckeridge, M.S. Cellulose crystals in fibrovascular bundles of sugarcane culms: orientation, size, distortion, and variability. Cellulose 19, 1507–1515 (2012). https://doi.org/10.1007/s10570-012-9743-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-012-9743-z