Abstract
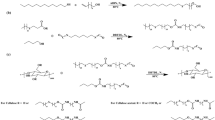
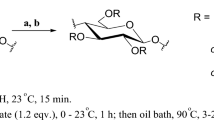
Amino cellulose sulfate (ACS); namely 6-deoxy-6-(ω-aminoethyl) amino cellulose-2,3(6)-O-sulfate (AECS) and 6-deoxy-6-(2-(bis-N′,N′-(2-aminoethyl)aminoethyl)) amino cellulose-2,3(6)-O-sulfate (BAECS) were prepared by a three step synthesis starting with the functionalization of microcrystalline cellulose with p-toluenesulfonyl (tosyl) groups (degree of substitution, DSTos between 0.55 and 1.37). Subsequently the introduction of the sulfate moieties was carried out (DSSulf between 1.09 and 1.27) and the tosyl groups at position 6 were replaced by a nucleophilic substitution reaction. As nucleophilic agents 1,2-diaminoethane and tris-(2-aminoethyl)amine were applied, yielding AECS (DSAEA values between 0.41 and 0.86) and BAECS (DSBAEA values between 0.32 and 0.74), respectively. The ACS samples were characterized by means of elemental analysis, 13C-NMR-, FT-IR-, and UV–Vis spectroscopy. Moreover, the solubility of the samples in water at different pH values and the molecular weights of the samples in aqueous solution were studied.




Similar content being viewed by others
References
Berlin P, Klemm D, Tiller J, Rieseler R (2000) A novel soluble aminocellulose derivative type: its transparent film-forming properties and its efficient coupling with enzyme proteins for biosensors. Macromol Chem Phys 201:2070–2082
Berlin P, Klemm D, Jung A, Liebegott H, Rieseler R, Tiller J (2003) Film-forming aminocellulose derivatives as enzyme-compatible support matrices for biosensor developments. Cellulose 10:343–367
Bieser AM, Tiller JC (2011) Mechanistic considerations on contact-active antimicrobial surfaces with controlled functional group densities. Macromol Biosci 11:526–534
Cakara D, Kleimann J, Borkovec M (2003) Microscopic protonation equilibria of poly(amidoamine) dendrimers from macroscopic titrations. Macromolecules 36:4201–4207
Ciferri A, Kudaibergenov S (2007) Natural and synthetic polyampholytes, 1 theory and basic structures. Macromol Rapid Commun 28:1953–1968
Dobrynin AV, Colby RH, Rubinstein M (2004) Polyampholytes. J Polym Sci B: Polym Phys 42:3513–3538
Fras Zemljič L, Čakara D, Michaelis N, Heinze T, Stana Kleinschek K (2011) Protonation behavior of 6-deoxy-6-(2-aminoethyl)amino cellulose: a potentiometric titration study. Cellulose 18:33–43
Gaweł K, Szczubiałka K, Zapotoczny S, Nowakowska M (2010) Zwitterionically modified hydroxypropylcellulose for biomedical applications. Eur Polym J 46:1475–1479
Genco T, Zemljič LF, Bračič M, Stana-Kleinschek K, Heinze T (2012) Physicochemical properties and bioactivity of a novel class of cellulosics: 6-deoxy-6-amino cellulose sulfate. Macromol Chem Phys 213:539–548
Gericke M, Liebert T, Heinze T (2009) Interaction of ionic liquids with polysaccharides, 8 – synthesis of cellulose sulfates suitable for polyelectrolyte complex formation. Macromol Biosci 9:343–353
Heinze T (1998) New ionic polymers by cellulose functionalization. Macromol Chem Phys 199:2341–2364
Heinze T, Koschella A (2005) Carboxymethyl ethers of cellulose and starch: a review. Macromol Symp 223:13–39
Heinze T, Liebert T (2001) Unconventional methods in cellulose functionalization. Prog Polym Sci 26:1689–1762
Heinze T, Rahn K (1996) The first report on a convenient synthesis of novel reactive amphiphilic polysaccharides. Macromol Rapid Commun 17:675–681
Heinze T, Liebert T, Koschella A (2006) Esterification of polysaccharides. Springer, Heidelberg
Heinze T, Nikolajski M, Daus S, Besong TMD, Michaelis N, Berlin P, Morris GA, Rowe AJ, Harding SE (2011) Protein-like oligomerization of carbohydrates. Angew Chem Int Ed 50:8602–8604
Jung A, Berlin P (2005) New water-soluble and film-forming aminocellulose tosylates as enzyme support matrices with Cu2+-chelating properties. Cellulose 12:67–84
Kudaibergenov SE, Ciferri A (2007) Natural and synthetic polyampholytes, 2 functions and applications. Macromol Rapid Commun 28:1969–1986
Liebert T, Heinze TJ (2001) Exploitation of reactivity and selectivity in cellulose functionalization using unconventional media for the design of products showing new superstructures. Biomacromolecules 2:1124–1132
Lowe AB, McCormick CL (2002) Synthesis and solution properties of zwitterionic polymers. Chem Rev 102:4177–4189
Nikolajski M, Wotschadlo J, Clement JH, Heinze T (2012) Amino functionalized cellulose nanoparticles: preparation, characterization and interactions with living cells. Macromol Biosci doi:10.1002/mabi.201200040
Petzold-Welcke K, Michaelis N, Heinze T (2009) Unconventional cellulose products through nucleophilic displacement reactions. Macromol Symp 280:72–85
Rahn K, Diamantoglou M, Klemm D, Berghmans H, Heinze T (1996) Homogeneous synthesis of cellulose p-toluenesulfonates in N,N-dimethylacetamide/LiCl solvent system. Angew Makromol Chem 238(1):143–163
Terbojevich M, Cosani A, Camilot M, Focher B (1995) Solution studies of cellulose tricarbanilates obtained in homogeneous phase. J Appl Polym Sci 55:1663–1671
Thielking H, Schmidt M (2006) Cellulose ethers. In: Ullmann’s encyclopedia of industrial chemistry, Wiley-VCH Verlag GmbH & Co. KGaA
Tiller J, Berlin P, Klemm D (1999) A novel efficient enzyme-immobilization reaction on NH2 polymers by means of l-ascorbic acid. Biotechnol Appl Biochem 30:155–162
Tiller J, Klemm D, Berlin P (2001) Designed aliphatic aminocellulose derivatives as transparent and functionalized coatings for enzyme immobilization. Des Monomers Polym 4:315–328
Zheng GZ, Meshitsuka G, Ishizu A (1995) Properties of an amphoteric cellulose derivative containing anionic carboxymethyl and cationic 2-hydroxy-3- (trimethylammonio) propyl substituents. J Polym Sci B: Polym Phys 33:867–877
Zheng GZ, Meshitsuka G, Ishizu A (1996) Interactions and chain mobilities of O-carboxymethyl-O-2-(diethylamino)ethylcellulose in aqueous solutions. Polymer 37:1629–1634
Acknowledgments
The research leading to these work received funding from European Community’s Seventh Framework program [FP7/2007-2013] under grant agreement no. 214015. We would like to thank Dr. Wolfgang Guenther (NMR measurements) and Dr. Grit Festag (SEC measurements), Institute of Organic Chemistry and Macromolecular Chemistry, Friedrich Schiller University Jena for their contributions.
Author information
Authors and Affiliations
Corresponding author
Additional information
T. Heinze et al.: Member of the European Polysaccharide Network of Excellence (EPNOE), http://www.epnoe.eu.
Rights and permissions
About this article
Cite this article
Heinze, T., Genco, T., Petzold-Welcke, K. et al. Synthesis and characterization of aminocellulose sulfates as novel ampholytic polymers. Cellulose 19, 1305–1313 (2012). https://doi.org/10.1007/s10570-012-9725-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10570-012-9725-1