Abstract
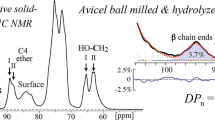
A homologous series of partially hydrolyzed celluloses (level-off-DP cellulose) with <italic>weight-average molecular weight</italic> (DP w ) < 150 were peracetylated and characterized by 1H-NMR spectroscopy. The results demonstrate the utility of 1H-NMR spectroscopy to assign the chemical shifts of all end groups of the peracetylated cellulose. On the one hand, the chemical shifts of all methine and methylene protons of the non-reducing terminal end group (TEG) as well as the α- and β-anomer of the reducing end group (REG) could be determined by two-dimensional NMR techniques (COSY-DQF) and by selective excitation of isolated proton signals (1D-TOCSY) of these end groups. On the other hand, the spectral resolution was high enough to determine the number-average molecular weight (DP n ) of peracetylated level-off-DP cellulose (LODP cellulose acetates) as shown in comparison with the data of gel permeation chromatography (GPC). This molecular weight determination of cellulose using end group analysis by means of 1H-NMR spectroscopy was demonstrated for the first time. Furthermore, a specific modification of hydroxyls in end groups could be exemplified in case of 1-OH-deacetylation of the REG of peracetylated LODP cellulose.
Similar content being viewed by others
References
O.A. Battista S. Coppiek J.A. Howsmon F.F. Morehead W.A. Sisson (1956) ArticleTitleLevel-off degree of polymerization Ind. Eng. Chem. 48 333–335
C.M. Buchanan J.A. Hyatt D.W. Lowman (1987) ArticleTitleTwo-dimensional NMR of polysaccharides: spectral assignment of cellulose triesters Macromolecules 20 2750–2754
C.M. Buchanan J.A. Hyatt D.W. Lowman (1988) ArticleTitleA simple n.m.r. method for assigning the carbonyl resonances of carbohydrate acetates Carbohydr. Res. 177 228–234
C.M. Buchanan J.A. Hyatt S.S. Kelly J.L. Little (1990) ArticleTitleα-d-Cellooligosaccharide acetate: physical and spectroscopic characterization and evaluation as models for cellulose triacetate Macromolecules 23 3747–3755
C. Deus H. Friebolin (1992) ArticleTitlePartially acetylated cellulose – synthesis and determination of the substitution distribution via proton NMR spectroscopy (in German) Makromol. Chem. 192 75–83
L.A. Flugge J.T. Blank P.A. Petillo (1999) ArticleTitleIsolation, modification, and NMR assignments of a series of cellulose oligomers J. Am. Chem. Soc. 121 7228–7238
D.Y. Gagnaire F.R. Taravel M.R. Vignon (1982) ArticleTitleTwo-dimensional spectroscopy: 1H-NMR of polysaccharides. Application to capsular heteroglycans and labeled cellulose triacetate Macromolecules 15 126–129
W.J. Goux C.J. Unkefer (1987) ArticleTitleThe assignment of carbonyl resonances in 13C-N.M.R. spectra of peracetylated mono- and oligo-saccharides containing d-glucose and d-mannose: an alternative method for structural determination of complex carbohydrates Carbohydr. Res. 159 191–210
K. Hikichi Y. Kakuta T. Katoh (1995) ArticleTitle1H-NMR study on substituent distribution of cellulose diacetates Polym. J. 27 659–663
E.A. Immergut B.G. Rånby (1956) ArticleTitleHeterogeneous acid hydrolysis of native cellulose fibers Ind. Eng. Chem. 48 1183–1189
K. Kamide K. Okajima K. Kowsaka M. Matsui (1987) ArticleTitleSolubility of cellulose acetate prepared by different methods and its correlationships with average acetyl group distribution on glucopyranose units Polym. J. 19 1405–1412
K. Kamide M. Saito (1994) ArticleTitleRecent advances in molecular and supermolecular characterization of cellulose and cellulose derivatives Macromol. Symp. 83 233–271
D. Klemm A. Stein T. Heinze B. Philipp W. Wagenknecht (1996) Cellulose-regioselectively substituted esters and ethers J.C. Salomone (Eds) The Polymeric Materials Encyclopedia: Synthesis, Properties and Applications, Vol. 2 CRC Press Boca Raton, FL 1043–1054
H. Kono Y. Numata N. Nagai T. Erata M. Takai (1999a) ArticleTitleCPMAS 13C NMR and X-ray studies of cellooligosaccharides acetates as a model for cellulose triacetate J. Polym. Sci., Polym. Chem. 37 4100–4107
H. Kono Y. Numata N. Nagai T. Erata M. Takai (1999b) ArticleTitleStudies of the series of cellooligosaccharide peracetates as a model for cellulose triacetate by C-13 CP/MAS NMR spectroscopy and X-ray analysis Carbohydr. Res. 322 256–263
Loth F. 1974. Investigations on preparation and characterization of cellulose pulvers and cellulose dispersions (in German). Ph.D. Thesis, Academy of Science of GDR, Teltow-Seehof, Germany.
T. Nishimura F. Nakatsubo (1997) ArticleTitleChemical synthesis of cellulose derivatives by a convergent synthetic method and several of their properties Cellulose 4 109–130
B. Pfannemüller T. Dengler (1988) ArticleTitleLinear and star-shaped hybrid polymers. 4. A new way for synthesis of cellulose and amylose containing block copolymers (in German) Makromol. Chem. 189 1965–1985
B. Philipp F. Loth (1975) ArticleTitleHeterogeneous hydrolysis of cellulose in various media of different swelling power (in German) Faserforsch. Textiltech. 26 415–420
A. Sharples (1958) ArticleTitleThe hydrolysis of cellulose and its relation to structure Trans. Faraday Soc. 54 913–917
M.M. Sim H. Kondo C.-H. Wong (1993) ArticleTitleSynthesis and use of glycosyl phosphites: An effective route to glycosyl phosphates, sugar nucleotides, and glycosides J. Am. Chem. Soc. 115 2260–2267
A. Stein D. Klemm (1995) ArticleTitleSelective derivatization of cellulose under heterogeneous reaction conditions and structure clarification by multi-dimensional NMR spectroscopy (in German) Das Papier 49 732–739
Y. Tezuka Y. Tsuchiya T. Shiomi (1996) ArticleTitle13C-NMR determination of substituent distribution in carboxymethyl cellulose by use of its peresterified derivatives Carbohydr. Res. 291 99–108
T. Yachi J. Hayashi M. Takai Y. Shimizu (1983) ArticleTitleSupramolecular structure of cellulose: stepwise decrease in LODP and particle size of cellulose hydrolyzed after chemical treatment J. Appl. Polym. Sci., Appl. Polym. Symp. 37 325–343
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Einfeldt, L., Günther, W., Klemm, D. et al. Peracetylated cellulose: end group modification and structural analysis by means of 1H-NMR spectroscopy. Cellulose 12, 15–24 (2005). https://doi.org/10.1007/s10570-004-5668-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10570-004-5668-5