Abstract
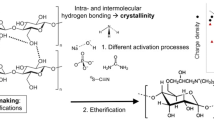
N-Methylmorpholine-N-oxide monohydrate (NMMO) is used as solvent for cellulose in the Lyocell process as a modern industrial fiber-making technology. Undesired chemical side reactions and byproduct formation in the system cellulose/NMMO/water are known to cause detrimental effects, such as chromophore formation and discoloration of the resulting fibers. A detailed kinetic study on the influence of carbonyl structures on chromophore formation in NMMO melts was carried out employing UV spectroscopy. Different sugar model compounds, such as reducing or non-reducing sugars, and sugars with additional oxidized functions, were applied. The chromophore formation rate differed widely for various reducing sugar model compounds, with pentoses generally reacting faster than hexoses, and carbohydrates with protected reducing end being largely inert. The effect of carbonyl groups on chromophore generation has been studied further using oligomers and oxidized pulps with different contents of carbonyl groups. As in the case of model compounds, also for the pulps a linear correlation between carbonyl content and chromophore formation rate was established. A distinct effect of hemicelluloses was observed.
Similar content being viewed by others
References
J. Buckingham (Eds) (1994) Dictionary of Natural Products Chapman & Hall London, UK
K.M. Biemel J. Conrad M.O. Lederer (2002) ArticleTitleUnexpected carbonyl mobility in aminoketoses: the key to major Mailard crosslinks Angew. Chem. Int. Ed. Engl. 41 IssueID5 801–804
J.P. Dworkin S.L. Miller (2000) ArticleTitleA kinetic estimate of the free aldehyde content of aldoses Carbohydr. Res. 329 359–365
H. Firgo M. Eibl W. Kalt G. Meister (1994) ArticleTitleCritical questions on the future of NMMO technology Lenz. Ber. 74 80–90
K. Heyns H. Paulsen (1962) ArticleTitleCatalytic oxidation of carbohydrates Adv. Carbohydr. Chem. 17 169–221
U. Mais H. Sixta (2004) ArticleTitleCharacterization of alkali-soluble hemicelluloses of hardwood dissolving pulps ACS Symp. Ser. 864 94–107
I. Marini H. Firgo W. Kalt (1994) ArticleTitleLenzing Lyocell Lenz. Ber. 74 53–57
G. Meister M. Wechsler (1998) ArticleTitleBiodegradation of N-methylmorpholine-N-oxide Biodegradation 9 IssueID2 91–102
J. Röhrling A. Potthast T. Rosenau T. Lange G. Ebner H. Sixta P. Kosma (2002a) ArticleTitleA novel method for the determination of carbonyl groups in cellulosics by fluorescence labeling. Part I: Method development Biomacromolecules 3 IssueID5 959–968
J. Röhrling A. Potthast T. Rosenau T. Lange A. Borgards H. Sixta P. Kosma (2002b) ArticleTitleA novel method for the determination of carbonyl groups in cellulosics by fluorescence labeling. Part II: Validation and applications Biomacromolecules 3 IssueID5 969–975
J. Röhrling A. Potthast T. Lange T. Rosenau I. Adorjan A. Hofinger P. Kosma (2002c) ArticleTitleSynthesis of oxidized 4-O-methyl-β-d-glucopyranoside and methyl β-d-glucopyranosyl-(1→4)-β-d-glucopyranoside derivatives as substrate for fluorescence labeling reactions Carbohydr. Res. 337 691–700
T. Rosenau A. Potthast H. Sixta P. Kosma (2001) ArticleTitleThe chemistry of side reactions and byproduct formation in the system NMMO/cellulose (Lyocell process) Prog. Polym. Sci. 26 1763–1837
T. Rosenau A. Potthast I. Adorjan A. Hofinger H. Sixta H. Firgo P. Kosma (2002a) ArticleTitleCellulose solutions in N-methylmorpholine-N-oxide (NMMO) – Degradation processes and stabilizers Cellulose 9 IssueID3–4 283–291
T. Rosenau A. Potthast A. Hofinger H. Sixta P. Kosma (2002b) ArticleTitleInstabilities in the system NMMO/water/cellulose (Lyocell process) caused by Polonowski type reactions Holzforschung 56 IssueID2 199–208
R. Schmid V.N. Sapunow (1982) Non-formal Kinetics Verlag Chemie GmbH WeinheimGermany
Sixta H., Schelosky N., Milacher W.,Baldinger T., Röder T. 2001Characterization of alkali-soluble pulp fractions by chromatography 11th ISWPC, June 11–14, 2001, NizzaFrance. Book of Abstracts Vol. 3 655--658
Y. Zhu J. Zajicek A.S. Serianni (2001) ArticleTitleAcyclic forms of [1-13C]aldohexoses in aqueous solution: quantitation by 13C NMR and deuterium isotope effects on tautomeric equilibria J. Org. Chem. 66 6244–6251
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Adorjan, I., Potthast, A., Rosenau, T. et al. Discoloration of cellulose solutions in N-methylmorpholine-N-oxide (Lyocell). Part 1: Studies on model compounds and pulps. Cellulose 12, 51–57 (2005). https://doi.org/10.1007/s10570-004-0212-1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s10570-004-0212-1