Abstract
This study explored the function of microRNAs (miRNAs) in invasive pituitary adenomas (IPA), and developed a microRNA-exosome strategy for the disease treatment. Differentially expressed miRNAs and tumor-associated markers in IPA, non-invasive pituitary adenoma (NIPA), and rat pituitary adenoma cells were identified by bioinformatics analysis and qRT-PCR. Then, the cells were treated by miR-149-5p and miR-99a-3p mimics or inhibitors, or incubated with modified exosome with overexpressed or silenced miRNAs. The cell behaviors were analyzed by molecular experiments. Xenograft assays were constructed by injection of pituitary adenoma cells and exosome into NU/NU nude mice. Tumor size, weight, and expressions of markers related to miRNAs and angiogenesis were determined. Target genes for miR-99a-3p and miR-149 were predicted and verified by bioinformatics analysis and molecular experiments. Twenty differentially expressed miRNAs were identified, among which miR-99a-3p and miR-149 were inhibited in both pituitary adenoma cells and tissues significantly. Expressions of E-cadherin and p53 were down-regulated, while those of MMP-2, MMP-9, N-cadherin, Vimentin, and VEGF were up-regulated in pituitary adenoma cells and tissues, especially in IPA. Further experiments revealed that overexpressed miR-149 and miR-99a-3p inhibited the growth and metastasis of pituitary adenoma cells and tube formation of endothelial cells. MiR-149 and miR-99a-3p overexpressed by exosome showed similar suppressive effects on cell viability, metastasis, tube formation ability, in vivo tumor growth, and expressions of angiogenesis-related markers. Further analysis showed that NOVA1, DTL, and RAB27B were targeted by miR-99a-3p. This study found that overexpressed miR-149-5p and miR-99a-3p induced by exosome could suppress the progression of IPA.
Graphical abstract and graphical headlights

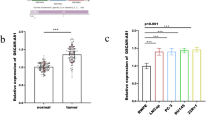
1. MiR-149-5p and miR-99a-3p affect the expression of EMT- and ECM-related markers and tumor-related genes in rat pituitary adenoma cells treated with exosomes.
2. Exosome inhibited the tumor growth.
3. Overexpressed miR-149-5p and miR-99a-3p induced by exosome







Similar content being viewed by others
References
Barrera CA, Cohen SA, Sankar WN, Ho-Fung VM, Sze RW, Nguyen JC. Imaging of developmental dysplasia of the hip: ultrasound, radiography and magnetic resonance imaging. Pediatr Radiol. 2019;49:1652–68. https://doi.org/10.1007/s00247-019-04504-3.
Becker A, Thakur BK, Weiss JM, Kim HS, Peinado H, Lyden D. Extracellular vesicles in cancer: cell-to-cell mediators of metastasis. Cancer Cell. 2016;30:836–48.
Bill M, Papaioannou D, Karunasiri M, Kohlschmidt J, Pepe F, Walker CJ, et al. Expression and functional relevance of long non-coding RNAs in acute myeloid leukemia stem cells. Leukemia. 2019;33:2169–82.
Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, et al. Rab27a supports exosome-dependent and -independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Cancer Res. 2012;72:4920–30.
Chen EB, et al. The miR-561-5p/CXCL1 signaling axis regulates pulmonary metastasis in hepatocellular carcinoma involving CXCR1 natural killer cells infiltration. Theranostics. 2019;9:4779–94.
Cheng A, et al. Helicobacter pylori causes epigenetic dysregulation of FOXD3 to promote gastric carcinogenesis. Gastroenterology. 2013;144:122–33.
Cui H, Wang Q, Lei Z, Feng M, Zhao Z, Wang Y, et al. DTL promotes cancer progression by PDCD4 ubiquitin-dependent degradation. J Experiment Clin Cancer Res. 2019;38:350.
D'angelo D, et al. RPSAP52 lncRNA is overexpressed in pituitary tumors and promotes cell proliferation by acting as miRNA sponge for HMGA proteins. J Mol Med (Berlin, Germany). 2019;97:1019–32.
De Majo F, De Windt LJ. RNA therapeutics for heart disease. Biochem Pharmacol. 2018;155:468–78.
Deng S, et al. Over-expressed miRNA-200b ameliorates ulcerative colitis-related colorectal cancer in mice through orchestrating epithelial-mesenchymal transition and inflammatory responses by channel of AKT2. Int Immunopharmacol. 2018;61:346–54. https://doi.org/10.1016/j.intimp.2018.06.024.
Di Ieva A, et al. MicroRNAs as biomarkers in pituitary tumors. Neurosurgery. 2014;75:181–9.
Diboun I, Wernisch L, Orengo CA, Koltzenburg M. Microarray analysis after RNA amplification can detect pronounced differences in gene expression using limma. BMC Genomics. 2006;7:252.
Dong W, et al. The RNA-binding protein RBM3 promotes cell proliferation in hepatocellular carcinoma by regulating circular RNA SCD-circRNA 2 production. EBioMedicine. 2019;45:155–67.
Dykes IM, Emanueli C. Transcriptional and post-transcriptional gene regulation by long non-coding RNA. Genom Proteom Bioinform. 2017;15:177–86.
Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, et al. The prevalence of pituitary adenomas: a systematic review. Cancer. 2004;101:613–9. https://doi.org/10.1002/cncr.20412.
Grzywa TM, et al. Lineage-dependent role of miR-410-3p as oncomiR in gonadotroph and corticotroph pituitary adenomas or tumor suppressor miR in somatotroph adenomas via MAPK, PTEN/AKT, and STAT3 signaling pathways. Endocrine. 2019;65:646–55.
He C, Wang L, Zhang J, Xu H. Hypoxia-inducible microRNA-224 promotes the cell growth, migration and invasion by directly targeting RASSF8 in gastric cancer. Mol Cancer. 2017;16:35.
Hu T, et al. miR-1 inhibits progression of high-risk papillomavirus-associated human cervical cancer by targeting G6PD. Oncotarget. 2016;7:86103–16. https://doi.org/10.18632/oncotarget.13344.
Hung MS, et al. Cul4A modulates invasion and metastasis of lung cancer through regulation of ANXA10. Cancers. 2019;11:E618.
Jeppesen DK, et al. Reassessment of exosome composition. Cell. 2019;177:428–45.
Ji D, Wang Y, Li H, Sun B, Luo X. Long non-coding RNA LINC00461/miR-149-5p/LRIG2 axis regulates hepatocellular carcinoma progression. Biochem Biophys Res Commun. 2019;512:176–81.
Kanlikilicer P, et al. Exosomal miRNA confers chemo resistance via targeting Cav1/p-gp/M2-type macrophage axis in ovarian cancer. EBioMedicine. 2018;38:100–12.
Kim MS, et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomed Nanotechnol Biol Med. 2016;12:655–64.
Lan X, et al. Whole-exome sequencing identifies variants in invasive pituitary adenomas. Oncol Lett. 2016;12:2319–28.
Li S, Luo W. Matrix metalloproteinase 2 contributes to aggressive phenotype, epithelial-mesenchymal transition and poor outcome in nasopharyngeal carcinoma. OncoTargets therapy. 2019;12:5701–11.
Li M, et al. Horizontal transfer of exosomal CXCR4 promotes murine hepatocarcinoma cell migration, invasion and lymphangiogenesis. Gene. 2018;676:101–9. https://doi.org/10.1016/j.gene.2018.07.018.
Liang Y, Wang W, Fang C, Raj SS, Hu WM, Li QW, et al. Clinical significance and diagnostic value of serum CEA, CA19-9 and CA72-4 in patients with gastric cancer. Oncotarget. 2016;7:49565–73. https://doi.org/10.18632/oncotarget.10391.
Liu C, et al. The role of FSCN1 in migration and invasion of pituitary adenomas. Mol Cell Endocrinol. 2016;419:217–24.
Lu B, Liu GL, Yu F, Li WJ, Xiang XX, Xiao HZ. MicroRNA-16/VEGFR2/p38/NF-κB signaling pathway regulates cell growth of human pituitary neoplasms. Oncol Rep. 2018;39:1235–44. https://doi.org/10.3892/or.2018.6227.
Ma C, et al. NAFLD causes selective CD4(+) T lymphocyte loss and promotes hepatocarcinogenesis. Nature. 2016;531:253–7. https://doi.org/10.1038/nature16969.
Melo SA, et al. Cancer exosomes perform cell-independent microRNA biogenesis and promote tumorigenesis. Cancer Cell. 2014;26:707–21.
Mishra M, Tiwari S, Gomes AV. Protein purification and analysis: next generation western blotting techniques. Expert Rev Proteomics. 2017;14:1037–53. https://doi.org/10.1080/14789450.2017.1388167.
Morrell ED, Kellum JA, Pastor-Soler NM, Hallows KR. Septic acute kidney injury: molecular mechanisms and the importance of stratification and targeting therapy. Crit Care (London, England). 2014;18:501. https://doi.org/10.1186/s13054-014-0501-5.
Morris DG, Musat M, Czirják S, Hanzély Z, Lillington DM, Korbonits M, et al. Differential gene expression in pituitary adenomas by oligonucleotide array analysis. Eur J Endocrinol. 2005;153:143–51. https://doi.org/10.1530/eje.1.01937.
Müssnich P, et al. Downregulation of miR-410 targeting the cyclin B1 gene plays a role in pituitary gonadotroph tumors. Cell cycle (Georgetown, Tex). 2015;14:2590–7.
Nieman LK, Biller BM, Findling JW, Murad MH, Newell-Price J, Savage MO, et al. Treatment of cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:2807–31. https://doi.org/10.1210/jc.2015-1818.
Rasheed SA, Teo CR, Beillard EJ, Voorhoeve PM, Zhou W, Ghosh S, et al. MicroRNA-31 controls G protein alpha-13 (GNA13) expression and cell invasion in breast cancer cells. Mol Cancer. 2015;14:67.
Roy S, et al. microRNA 193a-5p regulates levels of nucleolar- and spindle-associated protein 1 to suppress hepatocarcinogenesis. Gastroenterology. 2018;155:1951–1966.e1926.
Selman WR, Laws ER, Scheithauer BW, Carpenter SM. The occurrence of dural invasion in pituitary adenomas. J Neurosurg. 1986;64:402–7.
Shao Y, Liu X, Meng J, Zhang X, Ma Z, Yang G. MicroRNA-1251-5p promotes carcinogenesis and autophagy via targeting the tumor suppressor TBCC in ovarian cancer cells. Mol Therapy. 2019;27:1653–64.
Singh R, Pochampally R, Watabe K, Lu Z, Mo Y. Exosome-mediated transfer of miR-10b promotes cell invasion in breast cancer. Mol Cancer. 2014;13:256.
Thapar K, Kovacs K, Scheithauer BW, Stefaneanu L, Horvath E, Peter J. P, et al. Proliferative activity and invasiveness among pituitary adenomas and carcinomas: an analysis using the MIB-1 antibody. Neurosurgery. 1996;38:99–106.
Title AC, et al. Genetic dissection of the miR-200-Zeb1 axis reveals its importance in tumor differentiation and invasion. Nat Commun. 2018;9:4671.
Tripathi MK, Doxtater K, Keramatnia F, Zacheaus C, Yallapu MM, Jaggi M, et al. Role of lncRNAs in ovarian cancer: defining new biomarkers for therapeutic purposes. Drug Discov Today. 2018;23:1635–43.
Wang Y, Wen M, Kwon Y, Xu Y, Liu Y, Zhang P, et al. CUL4A induces epithelial-mesenchymal transition and promotes cancer metastasis by regulating ZEB1 expression. Cancer Res. 2014;74:520–31.
Wang B, Zhao H, Zhao L, Zhang Y, Wan Q, Shen Y, et al. Up-regulation of OLR1 expression by TBC1D3 through activation of TNFα/NF-κB pathway promotes the migration of human breast cancer cells. Cancer Lett. 2017;408:60–70.
Wang J, et al. ADAM12 induces EMT and promotes cell migration, invasion and proliferation in pituitary adenomas via EGFR/ERK signaling pathway. Biomed Pharmacother. 2018;97:1066–77.
Xiang F, et al. Ursolic acid reverses the chemoresistance of breast cancer cells to paclitaxel by targeting MiRNA-149-5p/MyD88. Front Oncol. 2019;9:501.
Xing B, Kong YG, Yao Y, Lian W, Wang RZ, Ren ZY. Study on the expression levels of CXCR4, CXCL12, CD44, and CD147 and their potential correlation with invasive behaviors of pituitary adenomas. Biomed Environ Sci. 2013;26:592–8.
Yadav S, Shekhawat M, Jahagirdar D, Kumar Sharma N. Natural and artificial small RNAs: a promising avenue of nucleic acid therapeutics for cancer. Cancer Biol Med. 2017;14:242–53.
Yamashita T, Takahashi Y, Takakura Y. Possibility of exosome-based therapeutics and challenges in production of exosomes eligible for therapeutic application. Biol Pharm Bull. 2018;41:835–42.
Yang X, Lei P, Wen T, Yihe H. A comparative study on two osteotomies in total hip arthroplasty for crowe type IV developmental dysplasia of the hip. Chin J Reparat Reconstr Surg. 2015;29:439–43.
Yang SY, Wei FL, Hu LH, Wang CL. PERK-eIF2alpha-ATF4 pathway mediated by endoplasmic reticulum stress response is involved in osteodifferentiation of human periodontal ligament cells under cyclic mechanical force. Cell Signal. 2016;28:880–6. https://doi.org/10.1016/j.cellsig.2016.04.003.
Yang J, et al. ZIP4 promotes muscle wasting and cachexia in mice with orthotopic pancreatic tumors by stimulating RAB27B-regulated release of extracellular vesicles from cancer cells. Gastroenterology. 2019;156:722–34.
Yildirim T, Guclu B, Karaguven D, Kaya A, Akan B, Cetin I. Cementless total hip arthroplasty in developmental dysplasia of the hip with end stage osteoarthritis: 2–7 years’ clinical results. Hip Int. 2015;25:442–6.
Yu YF, Zhang Y, Shen N, Zhang RY, Lu XQ. Effect of VEGF, P53 and telomerase on angiogenesis of gastric carcinoma tissue. Asian Pac J Trop Med. 2014;7:293–6.
Zhang YA, Liu HN, Jm Z, Zhang DY, Shen XZ, Liu TT. RNA binding protein Nova1 promotes tumor growth in vivo and its potential mechanism as an oncogene may due to its interaction with GABA Receptor-γ2. J Biomed Sci. 2016;23:71.
Zhang X, et al. Identification of miRNA-7 by genome-wide analysis as a critical sensitizer for TRAIL-induced apoptosis in glioblastoma cells. Nucleic Acids Res. 2017;45:5930–44.
Zhou Y, Zhou G, Tian C, Jiang W, Jin L, Zhang C, et al. Exosome-mediated small RNA delivery for gene therapy. Wiley Interdiscipl Rev RNA. 2016;7:758–71.
Funding
This work was supported by the Beijing Natural Science Foundation [7162034].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Figure 1
Venn diagram showed the overlaps of differentially expressed miRNAs in Normal, NIPA and IPA. Data (GSE46294) were downloaded from Gene Expression Omnibus Database. (PNG 325 kb)
Supplementary Figure 2
The effects of miR-149-5p and miR-99a-3p on viability, migration, invasion, colony formation of GH3 cells. (A) Expressions of miR-149-5p and miR-99a-3p in GH3 cells were detected by qRT-PCR. U6 snRNA served as a reference gene. (B) The viability of GH3 cells were determined by CCK-8 assay. (C) The migration ability of GH3 cells were determined by wound healing assay. bar=100 μm. 100 x magnification. (D) The invasion ability of GH3 cells were determined by transwell assay. bar=50 μm. 200 x magnification. (E) The cell proliferation abilities of GH3 cells were determined by colony formation assay. The colony formation rates were shown in the right. (F) The tube formation assay in HUVECs co-cultured with GH3 cells was shown in the left graphs. The relative tube length was calculated and shown in the right. bar =100 μm. 100 x magnification. **P<0.001 vs. NC. NC: negative control for mimic and inhibitor. Each experiment was independently repeated for three times. The data were shown as mean ± standard deviation. (PNG 1.59 mb)
Supplementary Figure 3
The identification of exosome secreted by rat pituitary adenoma cells and detection of associated genes expressions. (A) The morphological observation about exosome secreted by MMQ and GH3 cells using electron microscope. bar=200 μm. (B) Levels of exosome-associated markers, TSG101, Hsp70 and ALIX in MMQ cells, GH3 cells and the corresponding exosome determined by western blot. **P<0.001 vs. MMQ. ##P<0.001 vs. GH3. Each experiment was independently repeated for three times. The data were shown as mean ± standard deviation. (PNG 2.21 mb)
Supplementary Figure 4
The effects of exosome-drived miRNAs on viability, migration and invasion of GH3 cells. (A) Expressions of miR-149-5p and miR-99a-3p in GH3 cells were measured by qRT-PCR. *P<0.01,**P<0.001 vs. control. ^^P<0.001 vs. miR-149-5p inhibitor. ΔΔP<0.001 vs. miR-99a-3p mimics. (B) GH3 cells were treated by exosome secreted by MMQ after the corresponding transfection assay. The viability of GH3 cells was detected by CCK-8 assay. (C) The migration abilities of GH3 cells were measured by wound healing assay. Bar =100 μm. 100 x magnification. (D) The invasion abilities of GH3 cells were measured by transwell assay. Bar =50 μm. 200 x magnification. (E) The cell proliferation abilities of GH3 cells were then determined by colony formation assay. The colony formation rates were shown in the right. (F) The tube formation assay in HUVECs co-cultured by GH3 cells was shown in the left graphs. The relative tube length was calculated and shown in the right. bar=100 μm. 100 x magnification. **P<0.001 vs. Control. Each experiment was independently repeated for three times. The data were shown as mean ± standard deviation. (PNG 1.14 mb)
Supplementary Figure 5
Expressions of miR-149-5p, miR-99a-3p, MMP-2 and MMP-9, EMT-associated markers, tumor-associated genes in GH3 cells treated by exosome. (A-B) qRT-PCR analysis showed the expressions of miR-129-5p, miR-99a-3p, MMP-2 and MMP-9 in GH3 cells. (C-D) Levels of E-cadherin, N-cadherin and Vimentin in GH3 cells after treatment of exosome from MMQ cells were detected by western blot and qRT-PCR. (E-F) Levels of VEGF and p53 in MMQ cells after treatment of exosome from GH3 cells were detected by western blot and qRT-PCR. **P<0.001 vs. Control. ##P<0.001 vs. MMQ NC-exosome. Each experiment was independently repeated for three times. The data were shown as mean ± standard deviation. (PNG 2.25 mb)
Supplementary Figure 6
The effects of miR-149-5p and miR-99a-3p in exosome on tumor growth and tumor associated-genes expressions. (A) GH3 cells were subcutaneously injected into 8-week-old male NU/NU nude mice. Exosomes were injected into mice via tail vein. After 20 d of injection, tumor size and weight were analyzed in a Xenograft assay. 4 mice were in each group. (B) Expressions of miR-149-5p and miR-99a-3p in tumor tissues were detected by qRT-PCR. **P <0.001 vs. Control. ##P <0.001 vs. MMQ NC-exosome. (C) Levels of VEGF and p53 in tumor sections were determined by immunohistochemistry. bar =100 μm. 200 x magnification. Each experiment was independently repeated for three times. The data were shown as mean ± standard deviation. (PNG 600 kb)
Supplementary Figure 7
The target gene analysis of miR-149-5p and miR-99a-3p in pituitary adenoma. (A) Venn diagram showed the overlaps of the genes targeted by miR-149-5p and miR-99a-3p predicted by Targetscan 7.2 and DEGs in pituitary adenomas (GSE2175). (B) Expressions of predicted target genes for miR-149-5p and miR-99a-3p in GH3 cells were detected by qRT-PCR. GH3 cells were treated by exosome secreted by MMQ after the corresponding transfection assay. **P <0.001 vs. Control. Each experiment was independently repeated for three times. The data were shown as mean ± standard deviation. (PNG 1.11 mb)
Rights and permissions
About this article
Cite this article
Zhao, P., Cheng, J., Li, B. et al. Up-regulation of the expressions of MiR-149-5p and MiR-99a-3p in exosome inhibits the progress of pituitary adenomas. Cell Biol Toxicol 37, 633–651 (2021). https://doi.org/10.1007/s10565-020-09570-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-020-09570-0