Abstract
Purpose
Glucocorticoids (GCs) can facilitate bone formation, but prolonged GCs exposure in vivo can lead to osteoporosis. The mechanisms underlying these reciprocal effects have not been elucidated.
Methods
The epithelial sodium channel (ENaC) is a possible regulator of osteoblast proliferation and differentiation, so we examined whether ENaC was involved in mediating the effects of dexamethasone (Dex) on osteoblast.
Result
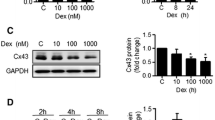
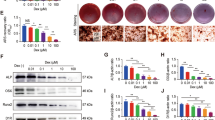
Expression of the functional α-ENaC subunit was upregulated by 10−8M and 10−6M Dex, but decreased by 10−4M Dex. Furthermore, Dex had similar dose-dependent effects on the expression of three osteogenic genes, Cbfa1, OPN, and OC, with low concentrations enhancing expression and higher concentrations suppressing expression. The effects of Dex on osteoblast proliferation, differentiation, and mineralization were examined in the presence and absence of the ENaC specific antagonist amiloride. Dex at 10−8M and 10−6M markedly increased osteoblast proliferation, alkaline phosphatase activity (an index of differentiation), and calcium nodule formation, while 10−4M had no effect or suppressed all these responses. Amiloride blocked the Dex-induced, osteoblast differentiation and mineralization but had no effect on osteoblast differentiation and mineralization when applied alone. But such changes did not show in osteoblast proliferation. However, the Dex-induced α-ENaC expression was not blocked by RU486, a GC receptor antagonist.
Conclusion
These results suggest that changes in ENaC activity may involved in Dex-induced differentiation and mineralization of osteoblast. But the Dex-induced effect on ENaC did not mediated by the GC genomic mechanism in osteoblast at this study.






Similar content being viewed by others
References
Almeida M, Han L, Ambrogini E, Weinstein RS, Manolagas SC (2011) Glucocorticoids and tumor necrosis factor (TNF) α increase oxidative stress and suppress WNT signaling in osteoblasts. J Biol Chem 286:44326-35
Azuma K, Urano T, Ouchi Y, Inoue S. Glucocorticoid-induced gene tripartite motif-containing 63(TRIM63) promotes differentiation of osteoblastic cells. Endocr J. 2010;57:455–62.
Bergann T, Zeissig S, Fromm A, Richter JF, Fromm M, Schulzke JD. Glucocorticoids and tumor necrosis factor-alpha synergize to induce absorption by the epithelial sodium channel in the colon. Gastroenterology. 2009;136:933–42.
Butterworth MB, Edinger RS, Frizzell RA, Johnson JP. Regulation of the epithelial sodium channel by membrane trafficking. Am J Physiol Ren Physiol. 2009;296:F10–24.
Caci E. Knockdown of ENaCα as a treatment for cystic fibrosis lung disease. Am J Respir Cell Mol Biol. 2009;40:211–6.
Dvorak-Ewell MM, Chen TH, Liang N, Garvey C, Liu B, Tu C, Chang W, Bikle DD, Shoback DM. Osteoblast extracellular Ca(2+) -sensing receptor regulates bone development, mineralization, and turnover. J Bone Miner Res. 2011;26:2935–47.
Eijken M, Koedam M, van Driel M, Buurman CJ, Pols HA, van Leeuwen JP. The essential role of glucocorticoids for proper human osteoblast differentiation and matrix mineralization. Mol Cell Endocrinol. 2006;248:87–93.
Gabusi E, Manferdini C, Grassi F, Piacentini A, Cattini L, Filardo G, Lambertini E, Piva R, Zini N, Facchini A, Lisignoli G (2011) Extracellular calcium chronically-induced human osteoblasts effects: specific modulation of osteocalcin and collagen type XV. J Cell Physiol 227:3151-3161
Gay CV, Gilman VR, Sugiyama T. Perspectives on osteoblast and osteoclast function. Poult Sci. 2000;79:1005–8.
Gu G, Hentunen TA, Nars M, Härkönen PL, Väänänen HK. Estrogen protects primary osteocytes against glucocorticoid-induced apoptosis. Apoptosis. 2005;10(3):583–95.
Hills CE, Squires PE, Bland R. Serum and glucocorticoid regulated kinase and disturbed renal sodium transport in diabetes. J Endocrinol. 2008;199:343–9.
Kim H-J. New understanding of glucocorticoid action in bone cells. BMB Rep. 2010;43:524–9.
Kalak R, Zhou H, Street J, Day RE, Modzelewski JR, Spies CM, Liu PY, Li G, Dunstan CR, Seibel MJ. Endogenous glucocorticoid signalling in osteoblasts is necessary to maintain normal bone structure in mice. Bone. 2009;45:61–7.
Kizer N, Guo XL, Hruska K. Reconstitution of stretch-activated cation channels by expression of the alpha-subunit of the epithelial sodium channel cloned from osteoblasts. Proc Natl Acad Sci U S A. 1997;94:1013–8.
Lane NE, Yao W. Glucocortid-induced bone fragility. Ann N Y Acad Sci. 2010;1192:81–3.
Li T, Folkesson HG. RNA interference for alpha-ENaC inhibits rat lung fluid absorption in vivo. Am J Physiol Lung Cell Mol Physiol. 2006;290:L649–60.
Li T, Koshy S, Folkesson HG. Involvement of falphagENaC and Nedd4–2 in the conversion from lung fluid secretion to fluid absorption at birth in the rat as assayed by RNA interference analysis. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1069–78.
Lian JB, Javed A, Zaidi SK, Lengner C, Montecino M, van Wijnen AJ, Stein JL, Stein GS. Regulatory controls for osteoblast growth and differentiation: role of Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr. 2004;14:1–11.
Liu L, Schlesinger PH, Slack NM, Friedman PA, Blair HC (2011) High capacity Na(+)/H(+) exchange activity in mineralizing osteoblasts. J Cell Physiol 226:1702-02
Lo YC, Chang YH, Wei BL, Huang YL, Chiou WF. Betulinic acid stimulates the differentiation and mineralization of osteoblastic MC3T3-E1 cells: involvement of BMP/Runx2 and β-catenin signals. J Agric Food Chem. 2010;58:6643–9.
Loffing J, Kaissling B. Sodium and calcium transport pathways along the mammalian distal nephron: from rabbit to human. Am J Physiol Ren Physiol. 2003;284:F628–43.
Makara GB, Haller J. Non-genomic effects of glucocorticoids in the neural system. Evidence, mechanisms and implications. Prog Neurobiol. 2001;65:367–90.
Mall MA. Role of the amiloride-sensitive epithelial Na+ channel in the pathogenesis and as a therapeutic target for cystic fibrosis lung disease. Exp Physiol. 2009;94:171–4.
Kimura M, Moteki H, Ogihara M. Inhibitory effects of dexamethasone on hepatocyte growth factor-induced DNA synthesis and proliferation in primary cultures of adult rat hepatocytes. Biol Pharm Bull. 2011;34(5):682–7.
Perlewitz A, Nafz B, Skalweit A, Fähling M, Persson PB, Thiele BJ. Aldosterone and vasipressin affect alpha- and gamma-ENaC mRNA translation. Nucleic Acid Res. 2010;38:5746–60.
Prager EM, Johnson LR. Stress at the synapse: signal transduction mechanisms of adrenal steroids at neuronal membranes. 2009; Sci Signal. 2: re5.
Satyanarayana R, Pondugula JD, Sanneman PW. Glucocorticoids stimulate cation absorption by semicircular canal duct epithelium via epithelial sodium channel. Am J Physiol Ren Physiol. 2004;10(286):1127–35.
Takahashi K, Saitoh A, Yamada M, Iwai T, Inagaki M, Yamada M. Dexamethasone indirectly induces Ndrg2 expression in rat astrocytes. J Neurosci Res. 2012;90(1):160–6.
Vachugova DV, Morachevskaid EA. Mechanosensitivity of cationic channels of DEG/ENaC family. Tsitologiia. 2009;51:806–14.
Boron WF. Medical physiology: a cellular and molecular approach. Elsevier: Saunders. 2005: 875–85.
Weinstein RS. Glucocorticoids, osteocytes, and skeletal fragility: the role of bone vascularity. Bone. 2010;46:564–70.
Yang GZ, Nie HG, Lu L, Chen J, Lu XY, Ji HL, Li QN. Estrogen regulates the expression and activity of epithelial sodium channel in mouse osteoblast. Cell Mol Biol. 2011;20:1480–6.
Acknowledgments
Appreciation is expression to the co-workers of the Department of Hematology, First Affiliated Hospital, Sun Yat-Sen University, for assistance with data analyses. This research is supported by the National Natural Science Foundation of China (30971172); and by the Guangdong Natural Science Fund (8251022401000005).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Lu, L., Wu, L., Jia, H. et al. The epithelial sodium channel is involved in dexamethasone-induced osteoblast differentiation and mineralization. Cell Biol Toxicol 28, 279–289 (2012). https://doi.org/10.1007/s10565-012-9222-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10565-012-9222-1