Abstract
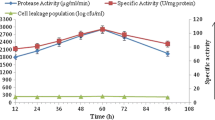
Immobilization represents one of the strategies to tackle the challenges related to the cost of production, storage stability, and property improvement of biocatalysts. In this study, the Bacillus cereus sp H1 α-amylase was immobilized using physical adsorption onto CaCO3 powder and entrapment in calcium alginate beads. Using experimental design, optimized yields of 80.77% and 97.42% were recorded for the adsorbed and encapsulated amylase, respectively. The surface-modified CaCO3 and Ca-alginate were characterized and confirmed by FTIR and SEM. After immobilization, an upgrade in pH and thermal stabilities was observed with the CaCO3-immobilized amylase compared to the free counterpart and Ca-alginate form. 65% or 52.45% of the activity was conserved after a 1-h incubation at pH 6.5–7.5 or 65°C, respectively. The Ca-alginate-immobilized amylase maintained over 60% of its activity in the presence of 5 mM Cu2+ and Mn2+, whereas the soluble enzyme’s activity decreased by 70%. Both immobilized biocatalysts maintained a minimum of 60% activity opposed to surfactants and inhibitors. Nevertheless, the Ca-alginate amylase demonstrated higher resistance to the commercial detergents, preserving at least 88% of its activity. Meanwhile, it retained over than 80% of activity after 35 days of storage at 4°C. The highest stability was observed after 2 reuses, with 55.11% residual activity when using CaCO3 as support. Furthermore, the obtained immobilized amylases were shown to be highly effective in removing starch stains from cotton fabrics, making them potent candidates for developing green and eco-friendly processes, especially for the textile industry.









Similar content being viewed by others
Data Availability
All the relevant data have been provided in the manuscript.
References
Tamborini L, Fernandes P, Paradisi F, Molinari F (2018) Flow Bioreactors as Complementary Tools for Biocatalytic Process Intensification. Trends Biotechnol 36:73–88. https://doi.org/10.1016/j.tibtech.2017.09.005
Zhu Y, Chen Q, Shao L et al (2019) Microfluidic immobilized enzyme reactors for continuous biocatalysis. Reaction Chem Eng 5:9–32. https://doi.org/10.1039/C9RE00217K
Reportlinker (2020) Enzymes Market Size, Share & Trends Analysis Report By Application, By Product, By Source, By Region And Segment Forecasts, 2020 - 2027. In: www.prnewswire.com. https://www.reportlinker.com/p05879568/?utm_source=PRN. Accessed 21 Dec 2023
Lee CH, Jin ES, Lee JH, Hwang ET (2020) Immobilization and Stabilization of Enzyme in Biomineralized Calcium Carbonate Microspheres. Frontiers in Bioengineering and Biotechnology 8: https://doi.org/10.3389/fbioe.2020.553591
Bashir N, Sood M, Bandral JD (2020) Enzyme immobilization and its applications in food processing: A review. International Journal of Chemical Studies 8:254–261. https://doi.org/10.22271/chemi.2020.v8.i2d.8779
Farias TC, Kawaguti HY, Bello Koblitz MG (2021) Microbial amylolytic enzymes in foods: Technological importance of the Bacillus genus. Biocatal Agric Biotechnol 35:102054. https://doi.org/10.1016/j.bcab.2021.102054
Ahmed NE, El Shamy AR, Awad HM (2020) Optimization and immobilization of amylase produced by Aspergillus terreus using pomegranate peel waste. Bulletin of the National Research Centre 44: https://doi.org/10.1186/s42269-020-00363-3
Singh R, Kumar M, Mittal A, Mehta PK (2016) Microbial enzymes: industrial progress in 21st century. 3 Biotech 6: https://doi.org/10.1007/s13205-016-0485-8
Cantone S, Ferrario V, Corici L et al (2013) Efficient immobilisation of industrial biocatalysts: criteria and constraints for the selection of organic polymeric carriers and immobilisation methods. Chem Soc Rev 42:6262. https://doi.org/10.1039/c3cs35464d
Madhavan A, Arun KB, Binod P et al (2021) Design of novel enzyme biocatalysts for industrial bioprocess: Harnessing the power of protein engineering, high throughput screening and synthetic biology. Biores Technol 325:124617. https://doi.org/10.1016/j.biortech.2020.124617
Garcia-Galan C, Berenguer-Murcia Á, Fernandez-Lafuente R, Rodrigues RC (2011) Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv Synth Catal 353:2885–2904. https://doi.org/10.1002/adsc.201100534
Guisan JM, López-Gallego F, Bolivar JM, Fernández-Lorente G (2020) The Science of Enzyme Immobilization. Methods Mol Biol 2100:1–26. https://doi.org/10.1007/978-1-0716-0215-7_1
Salem K, Jabalera Y, Puentes-Pardo JD et al (2021) Enzyme Storage and Recycling: Nanoassemblies of α-Amylase and Xylanase Immobilized on Biomimetic Magnetic Nanoparticles. ACS Sustain Chem Eng 9:4054–4063. https://doi.org/10.1021/acssuschemeng.0c08300
Maghraby YR, El-Shabasy RM, Ibrahim AH, Azzazy HME-S (2023) Enzyme Immobilization Technologies and Industrial Applications. ACS Omega 8:5184–5196. https://doi.org/10.1021/acsomega.2c07560
Yushkova ED, Nazarova EA, Matyuhina AV et al (2019) Application of Immobilized Enzymes in Food Industry. J Agric Food Chem 67:11553–11567. https://doi.org/10.1021/acs.jafc.9b04385
Almulaiky YQ, Khalil NM, Algamal Y et al (2023) Optimization of biocatalytic steps via response surface methodology to produce immobilized peroxidase on chitosan-decorated AZT composites for enhanced reusability and storage stability. Catal Lett 153(9):2543–2557
Al-Najada AR, Almulaiky YQ, Aldhahri M et al (2019) Immobilisation of α-amylase on activated amidrazone acrylic fabric: a new approach for the enhancement of enzyme stability and reusability. Sci Rep 9(1):12672
El-Shishtawy RM, Al Angari YM, Alotaibi MM et al (2023) Acrylic fabric and nanomaterials to enhance α-amylase-based biocatalytic immobilized systems for industrial food applications. Int J Biol Macromol 233:123539
Almulaiky YQ, Khalil NM, El-Shishtawy RM et al (2021) Hydroxyapatite-decorated ZrO2 for α-amylase immobilization: Toward the enhancement of enzyme stability and reusability. Int J Biol Macromol 167:299–308
Demir S, Gök SB, Kahraman MV (2011) α-Amylase immobilization on functionalized nano CaCO3by covalent attachment. Starch - Stärke 64:3–9. https://doi.org/10.1002/star.201100058
Al-Harbi SA, Almulaiky YQ (2020) Purification and biochemical characterization of Arabian balsam α-amylase and enhancing the retention and reusability via encapsulation onto calcium alginate/Fe2O3 nanocomposite beads. Int J Biol Macromol 160:944–952
Fernandez Caresani JR, Dallegrave A, dos Santos JHZ (2019) Amylases immobilization by sol–gel entrapment: application for starch hydrolysis. J Sol-Gel Sci Technol 94:229–240. https://doi.org/10.1007/s10971-019-05136-7
Nguyen HH, Kim M (2017) An Overview of Techniques in Enzyme Immobilization. Applied Sci Converg Technol 26:157–163. https://doi.org/10.5757/asct.2017.26.6.157
Ertan F, Yagar H, Balkan B (2007) Optimization of α-Amylase Immobilization in Calcium Alginate Beads. Prep Biochem Biotechnol 37:195–204. https://doi.org/10.1080/10826060701386679
Demirkan E, Dincbas S, Sevinc N, Ertan F (2011) Immobilization of B. amyloliquefaciens α-amylase and comparison of some of its enzymatic properties with the free form. Romanian Biotechnol Lett 16:6690–6701
Sharma A, Gupta G, Ahmad T, el al, (2021) Enzyme engineering: current trends and future perspectives. Food Rev Intl 37(2):121–154
Mahfoudhi A, Benmabrouk S, Fendri A, Sayari A (2022) Fungal lipases as biocatalysts: a promising platform in several industrial biotechnology applications. Biotechnol Bioeng 119:3370–3392. https://doi.org/10.1002/bit.28245
Kumar D, Bhardwaj R, Jassal S et al (2021) Application of enzymes for an eco-friendly approach to textile processing. Environ Sci Pollut Res 30:71838–71848. https://doi.org/10.1007/s11356-021-16764-4
Zafar A, Aftab MN, Iqbal I et al (2019) Pilot-scale production of a highly thermostable α-amylase enzyme from Thermotoga petrophila cloned into E. coli and its application as a desizer in textile industry. RSC Adv 9:984–992. https://doi.org/10.1039/c8ra06554c
Madhav S, Ahamad A, Singh P, Mishra PK (2018) A review of textile industry: Wet processing, environmental impacts, and effluent treatment methods. Environ Qual Manage 27:31–41. https://doi.org/10.1002/tqem.21538
Raghu HS, Rajeshwara NA (2015) Immobilization of α- Amylase (1, 4-α-D-Glucanglucanohydralase) by calcium alginate encapsulation. Int Food Res J 22:869–871
Gangadharan D, MadhavanNampoothiri K, Sivaramakrishnan S, Pandey A (2009) Immobilized bacterial α-amylase for effective hydrolysis of raw and soluble starch. Food Res Int 42:436–442
Salem K, Elgharbi F, Ben Hlima H, et al (2020) Biochemical characterization and structural insights into the high substrate affinity of a dimeric and Ca2+independentBacillus subtilisα‐amylase. Biotechnology Progress 36: https://doi.org/10.1002/btpr.2964
Phanphet S, Bangphan S (2021) Application of full factorial design for optimization of production process by turning machine. J Tianjin Univ Sci Technol 54:35–55. https://doi.org/10.17605/osf.io/3tesd
Zhang D, Wang R, Yang X (2009) Application of fractional factorial design to ZSM-5 synthesis using ethanol as template. Microporous Mesoporous Mater 126:8–13. https://doi.org/10.1016/j.micromeso.2009.03.015
Uzun Ü, Akatın MY (2019) Immobilization and some application of α-amylase purified from Rhizoctonia solani AG-4 strain ZB-34. Türk biyokimya dergisi 44:397–407. https://doi.org/10.1515/tjb-2018-0240
Horchani H, Bouaziz A, Gargouri Y, Sayari A (2012) Immobilized Staphylococcus xylosus lipase-catalysed synthesis of ricinoleic acid esters. J Mol Catal B Enzym 75:35–42. https://doi.org/10.1016/j.molcatb.2011.11.007
Montgomery DC (2017) Design and analysis of experiments. John Wiley & Sons Inc, Hoboken, Nj
Zusfahair Z, Riana Ningsih DRND, Kartika DKD et al (2017) Immobilization and Characterization of Bacillus Thuringiensis HCB6 Amylase in Calcium Alginate Matrix. Molekul 12:70. https://doi.org/10.20884/1.jm.2017.12.1.249
Won K, Kim S, Kim K-J et al (2005) Optimization of lipase entrapment in Ca-alginate gel beads. Process Biochem 40:2149–2154. https://doi.org/10.1016/j.procbio.2004.08.014
Zusfahair NDR, Kartika D et al (2020) Improved reuse and affinity of enzyme using immobilized amylase on alginate matrix. J Phys: Conf Ser 1494:012028. https://doi.org/10.1088/1742-6596/1494/1/012028
Nawawi NN, Hashim Z, Rahman RA et al (2020) Entrapment of porous cross-linked enzyme aggregates of maltogenic amylase from Bacillus lehensis G1 into calcium alginate for maltooligosaccharides synthesis. Int J Biol Macromol 150:80–89. https://doi.org/10.1016/j.ijbiomac.2020.02.032
Długosz O, Lis K, Banach M (2020) Synthesis and antimicrobial properties of CaCO3-nAg and nAg-CaCO3 nanocomposites. Nanotechnology 32(2):025715
Yang N, Wang R, Rao P et al (2019) The fabrication of calcium alginate beads as a green sorbent for selective recovery of Cu (II) from metal mixtures. Crystals 9(5):255
Ghamgui H, Miled N, Karra-chaâbouni M et al (2007) Immobilization studies and biochemical properties of free and immobilized Rhizopus oryzae lipase onto CaCO3: A comparative study. Biochem Eng J 37(1):34–41
Karim A, Nawaz MA, Aman A et al (2017) Role of anionic polysaccharide (alginate) on activity, stability and recycling efficiency of bacterial Endo (1→ 4) β-d-glucanase of GH12 family. Catal Lett 147:1792–1801
Kumar S, Haq I, Yadav A et al (2016) Immobilization and biochemical properties of purified xylanase from Bacillus amyloliquefaciens SK-3 and its application in kraft pulp biobleaching. J Clin Microbiol Biochem Technol 2(1):26–34
Riaz A, Ansari B, Siddiqui A et al (2015) Immobilization of α-amylase in operationally stable calcium-alginate beads: A cost effective technique for enzyme aided industrial processes. Int J Biotechnol Res 3:81–86
Kikani BA, Pandey S, Singh SP (2012) Immobilization of the α-amylase of Bacillus amyloliquifaciens TSWK1-1 for the improved biocatalytic properties and solvent tolerance. Bioprocess Biosyst Eng 36:567–577. https://doi.org/10.1007/s00449-012-0812-3
Dwevedi A (2016) Basics of enzyme immobilization. In: Enzyme immobilization. Springer, Cham, pp 21–44. https://doi.org/10.1007/978-3-319-41418-8_2
Kharrat N, Ali YB, Marzouk S et al (2011) Immobilization of Rhizopus oryzae lipase on silica aerogels by adsorption: Comparison with the free enzyme. Process Biochem 46:1083–1089. https://doi.org/10.1016/j.procbio.2011.01.029
Homaei A, Saberi D (2015) Immobilization of α-amylase on gold nanorods: An ideal system for starch processing. Process Biochem 50:1394–1399. https://doi.org/10.1016/j.procbio.2015.06.002
Herizi A, Rachid S, Djaffar D, Boubekeur N (2020) Optimization and Immobilization of alphaamylase from Bacillus subtilis in calcium alginate and calcium alginate – cellulosic residue beads. Microbiology Research 11: https://doi.org/10.4081/mr.2020.8458
Tüzmen N, Kalburcu T, Denizli A (2012) α-Amylase immobilization onto dye attached magnetic beads: Optimization and characterization. J Mol Catal B Enzym 78:16–23. https://doi.org/10.1016/j.molcatb.2012.01.017
Shukla RJ, Singh SP (2016) Structural and catalytic properties of immobilized α-amylase from Laceyella sacchari TSI-2. Int J Biol Macromol 85:208–216. https://doi.org/10.1016/j.ijbiomac.2015.12.079
Singh K, Srivastava G, Talat M et al (2015) α-Amylase immobilization onto functionalized graphene nanosheets as scaffolds: Its characterization, kinetics and potential applications in starch based industries. Biochem Biophysics Reports 3:18–25. https://doi.org/10.1016/j.bbrep.2015.07.002
Madhu A, Chakraborty JN (2017) Developments in application of enzymes for textile processing. J Clean Prod 145:114–133. https://doi.org/10.1016/j.jclepro.2017.01.013
Mojsov K (2012) Enzyme Scouring of Cotton Fabrics: A Review. Int J Marketing Technol 2:256–275
Sreelakshmi SN, Paul A, Vasanthi NS, Saravanan D (2013) Low-temperature acidic amylases fromAspergillusfor desizing of cotton fabrics. J Textile Institute 105:59–66. https://doi.org/10.1080/00405000.2013.810019
Chand N, Sajedi RH, Nateri AS et al (2014) Fermentative desizing of cotton fabric using an α-amylase-producing Bacillus strain: Optimization of simultaneous enzyme production and desizing. Process Biochem 49:1884–1888. https://doi.org/10.1016/j.procbio.2014.07.007
Sarethy IP, Saxena Y, Kapoor A et al (2013) Amylase produced by Bacillus sp. SI-136 isolated from sodic-alkaline soil for efficient starch desizing. J Biochem Technol 4:604–609
Şahinbaşkan BY, Kahraman MV (2010) Desizing of untreated cotton fabric with the conventional and ultrasonic bath procedures by immobilized and native α-amylase. Starch - Stärke 63:154–159. https://doi.org/10.1002/star.201000109
Dhingra S, Khanna M, Pundir C (2006) immobilization of alpha-amylase onto alkylamine glass beads affixed inside a plastic beaker. Indian J Chem Technol 13:119–121
Zdarta J, Meyer A, Jesionowski T, Pinelo M (2018) A General Overview of Support Materials for Enzyme Immobilization: Characteristics, Properties. Practical Utility Catalysts 8:92. https://doi.org/10.3390/catal8020092
Acknowledgements
This work is part of a doctoral thesis by Bouthaina Ben Hadj Hmida, whose research was financially supported by the Ministry of Higher Education and Scientific Research (Tunisia) through a grant to the Laboratory of Biochemistry and Enzymatic Engineering of Lipases-Engineering National School of Sfax (ENIS).
Author information
Authors and Affiliations
Contributions
Bouthaina Ben Hadj Hmida: Data curation, Formal analysis, Investigation, Methodology, Writing-Original Draft. Sameh Ben Mabrouk: Inversigation, Methodology, Writing—Review & Editing. Adel Sayari and Aida Hmida-Sayari: Conceptualization, Writing Review & Editing, Supervision, Project administration.
Corresponding author
Ethics declarations
Ethical Approval
Not applicable.
Consent to Publish
All authors have consent to publish the paper.
Consent to Participate
All authors have consent to participate in the study.
Statement of Informed Consent
The research does not involve human participants and animal experiments.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ben Hadj Hmida, B., Ben Mabrouk, S., Hmida-Sayari, A. et al. Optimization of Bacillus cereus sp H1 amylase immobilization for an eco-friendly approach to textile processing. Catal Lett (2024). https://doi.org/10.1007/s10562-024-04678-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10562-024-04678-y