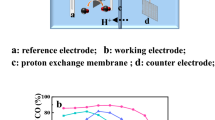
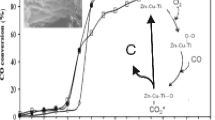
Abstract
The conversion of carbon dioxide to chemical raw materials or high value-added products can utilize the abundant carbon resources in nature as well as mitigate the greenhouse effect caused by excess carbon dioxide in the atmosphere. The electrocatalytic reduction of carbon dioxide process suffers from the problems of low product selectivity, low current efficiency, and poor electrode stability, and thus its application has been affected to some extent. In this study, bulk zinc-aluminum hydrotalcite (ZnAl-LDH) was prepared by the co-precipitation method, and the activity towards CO2 reduction was limited due to the stacking of the laminae, which led to the overlap of active sites. The electrochemical impedance was large and the electron transfer ability was poor, so it was thermally modified by means of calcination at different temperatures. Combined with the thermal stability of hydrotalcite, three representative temperatures, 300℃, 500℃ and 900℃ were selected, and the derived oxides obtained under calcination had different structures and thus possessed different activities towards CO2RR. Through electrochemical and physical characterizations, it was found that the ZnAl-LDH calcined at 900℃ was highly active for the electrocatalytic reduction of carbon dioxide, and the Faraday efficiency of carbon monoxide (FECO) of this material reached as high as 93% at a voltage of -1.05 V (vs RHE) with excellent electrochemical stability.
Graphical Abstract








Similar content being viewed by others
References
Johnson D, Qiao Z, Djire A (2021) Progress and challenges of carbon dioxide reduction reaction on transition metal based electrocatalysts. ACS Appl Energy Mater 4:8661–8684. https://doi.org/10.1021/acsaem.1c01624
Nguyen TN, Guo J, Sachindran A, Li F, Seifitokaldani A, Dinh CT (2021) Electrochemical CO2 reduction to ethanol: from mechanistic understanding to catalyst design. J Mater Chem A 9:12474–12494. https://doi.org/10.1039/D1TA01115D
Wei L, Li R, Kong W, Tan P, Fan Q, Yu C (2022) Highly selective electrocatalytic reduction of carbon dioxide to ethylene on CuCl-derived Cu. Mater Chem Phys 291:126660. https://doi.org/10.1016/j.matchemphys.2022.126660
Lee S, Choi M, Lee J (2020) Looking back and looking ahead in electrochemical reduction of CO2. Chem Rec 20:89–101. https://doi.org/10.1002/tcr.201900048
Roy A, Jadhav HS, Park SJ, Seo JG (2021) Recent advances in the possible electrocatalysts for the electrochemical reduction of carbon dioxide into methanol. J Alloys Compd 887:161449. https://doi.org/10.1016/j.jallcom.2021.161449
Yaashikaa P, Kumar PS, Varjani SJ, Saravanan A (2019) A review on photochemical, biochemical and electrochemical transformation of CO2 into value-added products. J CO2 Util 33:131–147. https://doi.org/10.1016/j.jcou.2019.05.017
Cui Y, He B, Liu X, Sun J (2020) Ionic liquids-promoted electrocatalytic reduction of carbon dioxide. Ind Eng Chem Res 59:20235–20252. https://doi.org/10.1021/acs.iecr.0c04037
Lu X, Tu W, Zhou Y, Zou Z (2023) Effects of Electrolyte Ionic Species on Electrocatalytic Reactions: Advances, Challenges, and Perspectives. Adv Energy Mater 13:2300628. https://doi.org/10.1002/aenm.202300628
Zhang X, Guo SX, Gandionco KA, Bond AM, Zhang J (2020) Electrocatalytic carbon dioxide reduction: From fundamental principles to catalyst design. Mater Today Adv 7:100074. https://doi.org/10.1016/j.mtadv.2020.100074
Wang C, Cao M, Jiang X, Wang M, Shen Y (2018) A catalyst based on copper-cadmium bimetal for electrochemical reduction of CO2 to CO with high faradaic efficiency. Electrochim Acta 271:544–550. https://doi.org/10.1016/j.electacta.2018.03.156
Wang N, Liu Z, Ma J et al (2020) Sustainability perspective-oriented synthetic strategy for zinc single-atom catalysts boosting electrocatalytic reduction of carbon dioxide and oxygen. ACS Sustain Chem Eng 8:13813–13822. https://doi.org/10.1021/acssuschemeng.0c05158
Zhao Y, Zheng L, Jiang D et al (2021) Nanoengineering metal–organic framework-based materials for use in electrochemical CO2 reduction reactions. Small 17:2006590. https://doi.org/10.1002/smll.202006590
Hongrutai N, Watmanee S, Pinthong P, Panpranot J (2022) Electrochemical reduction of carbon dioxide on the oxide-containing electrocatalysts. J CO2 Util 64:102194. https://doi.org/10.1016/j.jcou.2022.102194
Rutkowska IA, Wadas A, Szaniawska E, Chmielnicka A, Zlotorowicz A, Kulesza PJ (2020) Elucidation of activity of copper and copper oxide nanomaterials for electrocatalytic and photoelectrochemical reduction of carbon dioxide. Curr Opin Electrochem 23:131–138. https://doi.org/10.1016/j.coelec.2020.05.014
Nguyen DL, Kim Y, Hwang YJ, Won DH (2020) Progress in development of electrocatalyst for CO2 conversion to selective CO production. Carbon Energy 2:72–98. https://doi.org/10.1002/cey2.27
Liu K, Wang J, Shi M, Yan J, Jiang Q (2019) Simultaneous achieving of high faradaic efficiency and CO partial current density for CO2 reduction via robust, noble-metal-free Zn nanosheets with favorable adsorption energy. Adv Energy Mater 9:1900276. https://doi.org/10.1002/aenm.201900276
Dong W, Zhong DZ, Hao GY, Li JP, Qiang Z (2021) ZnOHF nanorods for efficient electrocatalytic reduction of carbon dioxide to carbon monoxide. J Fuel Chem Technol 49:1379–1388. https://doi.org/10.1016/S1872-5813(21)60082-8
Yan LG, Yang K, Shan RR, Yu HQ, Du B (2015) Calcined ZnAl-and Fe3O4/ZnAl–layered double hydroxides for efficient removal of Cr (VI) from aqueous solution. RSC Adv 5:96495–96503. https://doi.org/10.1039/C5RA17058C
Vu VN, Pham THT, Nguyen QD et al (2022) Enhanced Adsorption, Photocatalytic Degradation Efficiency of Phenol Red Using CuZnAl Hydrotalcite Synthesized by Co-Precipitation Technique. Processes 10:1555. https://doi.org/10.3390/pr10081555
Abbasi M, Sabzehmeidani MM, Ghaedi M, Jannesar R, Shokrollahi A (2021) Facile fabrication of leaf coral-like structured Cu-Al LDH/PVDF composite adsorptive membrane with enhanced adsorption performance. Mater Sci Eng B 267:115086. https://doi.org/10.1016/j.mseb.2021.115086
Zhang H, Dai Z, Sui Y et al (2018) Scavenging of U (VI) from impregnated water at uranium tailings repository by tripolyphosphate intercalated layered double hydroxides. Ind Eng Chem Res 57:17318–17327. https://doi.org/10.1021/acs.iecr.8b04636
Zhao G, Zou J, Chen X, Yu J, Jiao F (2020) Layered double hydroxides materials for photo (electro-) catalytic applications. Chem Eng J 397:125407. https://doi.org/10.1016/j.cej.2020.125407
Bankauskaite A, Baltakys K (2015) Thermal, texture and reconstruction properties of hydrotalcites substituted with Na+ or K+ ions. J Therm Anal Calorim 121:227–233. https://doi.org/10.1007/s10973-015-4499-y
Yang Y, Yang M, Zheng Z, Zhang X (2020) Highly effective adsorption removal of perfluorooctanoic acid (PFOA) from aqueous solution using calcined layer-like Mg-Al hydrotalcites nanosheets. Environ Sci Pollut Res 27:13396–13408. https://doi.org/10.1007/s11356-020-07892-4
Yuan D, Zhou L, Fu D (2017) Adsorption of methyl orange from aqueous solutions by calcined ZnMgAl hydrotalcite. Appl Phys A 123:1–8. https://doi.org/10.1007/s00339-017-0763-2
Jung IK, Jo Y, Han SC, Yun JI (2020) Efficient removal of iodide anion from aqueous solution with recyclable core-shell magnetic Fe3O4@ Mg/Al layered double hydroxide (LDH). Sci Total Environ 705:135814. https://doi.org/10.1016/j.scitotenv.2019.135814
Khalkhali M, Zhu X, Shi Y, Liu Q, Choi P, Zhang H (2020) Structure and CO2 physisorption capacity of hydrotalcite-derived oxide. J CO2 Util 36:64–75. https://doi.org/10.1016/j.jcou.2019.10.019
Choi Y, Jung H, Kim S, Han JW, Lee KB (2022) Structural changes of hydrotalcite-based Co-containing mixed oxides with calcination temperature and their effects on NOx adsorption: A combined experimental and DFT study. Chem Eng J 437:135209. https://doi.org/10.1016/j.cej.2022.135209
Li L, Yang J, Li L, Huang Y, Zhao J (2022) Electrolytic reduction of CO2 in KHCO3 and alkanolamine solutions with layered double hydroxides intercalated with gold or copper. Electrochim Acta 402:139523. https://doi.org/10.1016/j.electacta.2021.139523
Fu Y, Chen L, Xiong Y et al (2022) NiFe-CN catalysts derived from the solid-phase exfoliation of NiFe-layered double hydroxide for CO2 electroreduction. New J Chem 46:16019–16024. https://doi.org/10.1039/D2NJ02234F
Zhang B, Zhang J (2017) Rational design of Cu-based electrocatalysts for electrochemical reduction of carbon dioxide. J Energy Chem 26:1050–1066. https://doi.org/10.1016/j.jechem.2017.10.011
Zhao Q, Ge Y, Fu K, Ji N, Song C, Liu Q (2018) Oxidation of acetone over Co-based catalysts derived from hierarchical layer hydrotalcite: Influence of Co/Al molar ratios and calcination temperatures. Chemosphere 204:257–266. https://doi.org/10.1016/j.chemosphere.2018.03.198
Dongare S, Singh N, Bhunia H, Bajpai PK (2021) Electrochemical reduction of CO2 using oxide based Cu and Zn bimetallic catalyst. Electrochim Acta 392:138988. https://doi.org/10.1016/j.electacta.2021.138988
Cai Y, Ma Y, Feng J et al (2020) Insight into the performance and mechanism of low-cost phytic acid modified Zn-Al-Ti LMO for U(VI) removal. Chem Eng J 402:125510. https://doi.org/10.1016/j.cej.2020.125510
Kang M, Zhou H, Tang D, Chen X, Guo Y, Zhao N (2019) Methyl N-phenyl carbamate synthesis over Zn/Al/Ce mixed oxide derived from hydrotalcite-like precursors. RSC Adv 9:42474–42480. https://doi.org/10.1039/C9RA09642F
Zhang T, Yuan B, Wang W, He J, Xiang X (2023) Tailoring* H Intermediate Coverage on the CuAl2O4/CuO Catalyst for Enhanced Electrocatalytic CO2 Reduction to Ethanol. Angew Chem 135:e202302096. https://doi.org/10.1002/ange.202302096
Fan X, Yang Z, Wen R, Yang B, Long W (2013) The application of Zn–Al-hydrotalcite as a novel anodic material for Ni–Zn secondary cells. J Power Sources 224:80–85. https://doi.org/10.1016/j.jpowsour.2012.09.101
Chen X, Yang Z, Wang L, Qin H, Tong M (2019) Synthesis of Hollow Spherical Zinc-Aluminum Hydrotalcite and Its Application as Zinc Anode Material. J Electrochem Soc 166:A2589. https://doi.org/10.1149/2.1141912jes
Chen CR, Zeng HY, Xu S, Liu XJ, Duan HZ, Han J (2017) Preparation of mesoporous material from hydrotalcite/carbon composite precursor for chromium (VI) removal. J Taiwan Inst Chem Eng 70:302–310. https://doi.org/10.1016/j.jtice.2016.10.018
López-Muñoz B, Iturbe-García J, Olguin M (2019) Physicochemical properties of layered double hydroxides by-product obtained from Al–MgO mechanochemical process to produce hydrogen. Chem Pap 73:415–424. https://doi.org/10.1007/s11696-018-0602-8
Ren D, Ang BSH, Yeo BS (2016) Tuning the selectivity of carbon dioxide electroreduction toward ethanol on oxide-derived CuxZn catalysts. ACS Catal 6:8239–8247. https://doi.org/10.1021/acscatal.6b02162
Tan F, Liu T, Liu E, Zhang Y (2024) On ZnAlCe-THs Nanocomposites Electrocatalysts for Electrocatalytic Carbon Dioxide Reduction to Carbon Monoxide. Catal Lett 154:11–22. https://doi.org/10.1007/s10562-023-04302-5
Zhang ZY, Tian H, Bian L, Liu SZ, Liu Y, Wang ZL (2023) Cu-Zn-based alloy/oxide interfaces for enhanced electroreduction of CO2 to C2+ products. J Energy Chem 83:90–97. https://doi.org/10.1016/j.jechem.2023.04.034
Luo W, Zhang Q, Zhang J, Moioli E, Zhao K, Züttel A (2020) Electrochemical reconstruction of ZnO for selective reduction of CO2 to CO. Appl Catal B Environ 273:119060. https://doi.org/10.1016/j.apcatb.2020.119060
Guo W, Shim K, Kim YT (2020) Ag layer deposited on Zn by physical vapor deposition with enhanced CO selectivity for electrochemical CO2 reduction. Appl Surf Sci 526:146651. https://doi.org/10.1016/j.apsusc.2020.146651
Wang M, Ren X, Yuan G et al (2020) Selective electroreduction of CO2 to CO over co-electrodeposited dendritic core-shell indium-doped Cu@ Cu2O catalyst. J Cu2O Util 37:204–212. https://doi.org/10.1016/j.jcou.2019.12.013
Ye Y, Liu Y, Li Z, Zou X, Wu H, Lin S (2021) Highly selective and active Cu-In2O3/C nanocomposite for electrocatalytic reduction of CO2 to CO. J Colloid Interface Sci 586:528–537. https://doi.org/10.1016/j.jcis.2020.10.118
Funding
We gratefully appreciate the support of the National Natural Science Foundation of China (No. 51208299).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflicts of interest regarding the publication of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, L., Gao, Y., Yu, S. et al. Faster Kinetics and High Selectivity for Electrolytic Reduction of CO2 with Zn0/Zn2+ Interface of ZnO/ZnAl2O4 Derived from Hydrotalcite. Catal Lett (2024). https://doi.org/10.1007/s10562-024-04648-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10562-024-04648-4