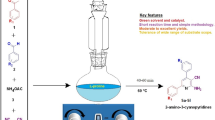
Abstract
The unique physicochemical properties of PEG-400 were imparted to (L)-prolinamide to afford a homogeneous, solvent-free, recyclable organocatalyst for scalable enantioselective aldol reaction between acetone and benzaldehyde to afford (R)-4-hydroxy-4-phenylbutan-2-one. The reaction was scaled up to afford 41 g of ketol in 95% yield and an ee of 91% using 30 mol% catalyst, 10 mol% trifluoroacetic acid (TFA), and 135 equivalent moles of acetone with respect to benzaldehyde. The catalyst was recycled 4 times with no obvious loss of activity. The role of PEG is discussed at the molecular level. This type of catalyst may provide new possibilities for the so-far-thwarted attempts at large-scale application of proline organocatalysis to asymmetric aldol reactions.
Graphical Abstract






Similar content being viewed by others
References
Ferré M, Pleixats R, Wong Chi Man M, Cattoën X (2016) Recyclable organocatalysts based on hybrid silicas. Green Chem 18:881–922
Agai T, Tanaka S, Dkawa S (1943) J Pharm Soc Jap 63:269
Karimian K, Mohanazadeh F, Rezai S (1983) Application of polymer-bound thiazolium salts to the synthesis of acyloins and benzoins: effects of solvent and substituents of the thiazolium nucleus. J Heterocyclic Chem 20:1119
Haghighi AJ, Mokhtari J, Karimian K (2020) N PEGylated thiazolium salt: a green and reusable homogenous organocatalyst for the synthesis of benzoins and acyloins. Catal Lett 151:1646–1652
Gaggero N, Pandini S (2017) Advances in chemoselective intermolecular cross benzoin-type condensation reactions. Org Biomol Chem 15:6867–6887
Stetter H (1976) Catalyzed addition of aldehydes to activated double bonds—a new synthetic approach. Angew Chem Int Ed Engl 15:639–712
Wurz NE, Daniliuc CG, Glorius F (2012) Highly enantioselective intermolecular stetter reaction of simple acrylates: synthesis of α-chiral γ-ketoesters. Chem Eur J 18:16297
Christmann M (2005) New developments in the asymmetric Stetter reaction. Angew Chem Int Ed 44:2632–2634
List B, Lerner RA, Barbas CF III (2000) Proline-catalyzed direct asymmetric aldol reactions. J Am Chem Soc 122:2395–2396
Adamu A, Aliyu F, Huyop F, Roswanira A, Wahab RA (2018) Molecular basis and engineering of enzymes stereospecificity. J Mol Biol Methods 1:1–7
Chan CHS, Pan L, Yi LY, Yuan S (2018) Rationalization of stereoselectivity in enzyme reactions. WIREs Comput Mol Sci 9:e1403
Ruipu M, Wang Z, Wamsley MC, Duke CN, Lii PH, Epley SE, Todd LC, Roberts PJ (2020) Application of enzymes in regioselective and stereoselective organic reactions. Catalysts 10:832–856
Chapman J, Ismail AI, Dinu CZ (2018) Industrial applications of enzymes: recent advances. Techn Outlooks, Catal 8:238
Sheldon RA, Bradya D, Bode ML (2020) The hitchhiker’s guide to biocatalysis: recent advances in the use of enzymes in organic synthesis. Chem Sci 11:2587–2605
Hanefeld U, Hollmann F, Paul CE (2022) Biocatalysis making waves in organic chemistry. Chem Soc Rev 51:594–627
Nemat-Gorgani M, Karimian K, Mohanazadeh F (1985) Synthesis, characterization, and properties of hexadecyl silica: a novel hydrophobic matrix for protein immobilization. J Am Chem Soc 107:4757–4759
Nemat-Gorgani M, Karimian K (1982) Non-ionic adsorptive immobilization of proteins to palmityl-substituted sepharose 4b. Eur J Biochem 123:601–610
Guerrieri A, Ciriello R, Bianco G, De Gennaro F, Frascaro S (2020) allosteric enzyme-based biosensors—kinetic behaviors of immobilized l-lysine-α-oxidase from Trichoderma viride: ph influence and allosteric properties. Biosensors 10:145–158
Oliveira VG, Cardoso MFC, Forezi LSM (2018) Organocatalysis: a brief overview on its evolution and applications. Catalysts 8:605–634
Branco LC, Phillips AMF, Marques MM, Gago S, Branco PS (2016) Recent advances in sustainable organocatalysis. In: Srour H (ed) Recent advances in organocatalysis. Intech, pp 141–182
Torres RR (2013) Stereoselective organocatalysis bond formation methodologies and activation modes. John Wiley & Sons, Inc., Hoboken, pp 29–588
Vachana BS, Karuppasamya M, Vinotha P, Sridharana V, Menendez JC (2020) Stereoselective organic synthesis in water: organocatalysis by proline and its derivatives. In: Inamuddin RB, Asiri AM (eds) Green sustainable process for chemical and environmental engineering and science. Elsevier, Cambridge, pp 191–228
Cobb AJA, Shaw DA, Longbottom DA, Gold JB, Ley SV (2005) Organocatalysis with proline derivatives: improved catalysts for the asymmetric Mannich, nitro-Michael and aldol reactions. Org Biomol Chem 3:84–96
Mahrwald R (2011) Enantioselective organocatalyzed reactions II. Springer, Dordrecht Heidelberg, p 84
Benaglia M, Puglisi A (2020) Catalyst immobilization methods and applications. Wiley-VCH Verlag GmbH, Weinheim
Reddy RJ, Chen K (2016) Other 2-substituted pyrrolidines as asymmetric organocatalysts. In: Clark J (ed) sustainable catalysis without metals or other endangered elements part 1. The Royal Society of Chemistry, Cambridge, pp 200–235
Sagamanova IK, Sayalero S, Martínez-Arranz S, Albéniz AC, Pericàs MA (2015) Asymmetric organocatalysts supported on vinyl addition polynorbornenes for work in aqueous media. Catal Sci Technol 3:754–764
Pedrosa R, Andre JM (2016) Prolinamides as asymmetric organocatalysts. In: Clark J (ed) Sustainable catalysis without metals or other endangered elements Part 1. Royal Society of Chemistry, Cambridge, pp 120–133
Enders D, Httl MRM, Runsink J, Raabe G, Wendt B (2007) Organocatalytic one-pot asymmetric synthesis of functionalized tricyclic carbon frameworks from a triple-cascade/diels–alder sequence. Angew Chem Int Ed 46:467–469
Grondal C, Jeant M, Enders D (2010) Organocatalytic cascade reactions as a new tool in total synthesis. Nat Chem 2:167–178
Gryko D, Chromiński M, Pielacińska DJ (2011) Prolinethioamides versus prolinamides in organocatalyzed aldol reactions—a comparative study. Symmetry 3:265–282
Arslan N, Ercan S, Pirinççioğlu N (2020) Proline-based organocatalyst-mediated asymmetric aldol reaction of acetone with substituted aromatic aldehydes: an experimental and theoretical study. Turk J Chem 4:335–351
Naresh T, Kumar TP, Haribabu K, Chandrasekhar S (2014) AZT-prolinamide: the nucleoside derived pyrrolidine catalysts or asymmetric aldol reactions using water as solvent. Tetrahedron Asymmetry 25:1340–1345
Huang SH, Wang H-J, Shi J (2010) Theoretical study on acidities of (S)-prolinamide derivatives in DMSO and its implications for organocatalysis. J Phys Chem A 114:1068–1081
Henriksen NE, Hansen FY (2019) Theories of molecular reaction dynamics the microscopic foundation of chemical kinetics, 2nd edn. Oxford University Press, New York
Maya V, Raj M, Singh VK (2007) Highly enantioselective organocatalytic direct aldol reaction in an aqueous medium. Org Lett 9:2593–2595
Clemente FR, Houk KN (2004) Computational evidence for the enamine mechanism of intramolecular aldol reactions catalyzed by proline. Angew Chem 116:5889–5892
Bahmanyar S, Houk KN, Martin HJ, List B (2003) Quantum mechanical predictions of the stereoselectivities of proline-catalyzed asymmetric intermolecular aldol reactions. J Am Chem Soc 125:2475–2479
Angelici G, Corrêa RJ, Garden SJ, Tomasini C (2009) Water influences the enantioselectivity in the proline or prolinamide-catalyzed aldol addition of acetone to isatins. Tetrahedron Lett 50:814–817
Aratake S, Itoh T, Okano T, Nagae N, Sumija T, Shoji M, Hayashi Y (2007) Highly diastereo- and enantioselective direct aldol reactions of aldehydes and ketones catalyzed by siloxyproline in the presence of water. Chem Eur J 13:10246–10256
Michelet B, Martin-Mingot A, Rodriguez J, Thibaudeau S, Bonne D (2023) Enantioselective organocatalysis and superacid activation: challenges and opportunities. Chem Eur J 29:e202300440
Hajipour AR, Khorsandi Z (2017) Application of immobilized proline on CNTs and proline ionic liquid as novel organocatalysts in the synthesis of 2-amino-4H-pyran derivatives: a comparative study between their catalytic activities. Chem Select 2:8976–8982
Bartók M (2015) Advances in immobilized organocatalysts for the heterogeneous asymmetric direct aldol reactions. Catal Rev: Sci Eng 57:192–255
Aukland MH, List B (2021) Organocatalysis emerging as a technology. Pure Appl Chem 93:2–11
Chandrasekhar S, Narsihmulu C, Sultana SS, Reddy NR (2002) Poly(ethylene glycol (PEG) as a reusable solvent medium for organic synthesis application in the heck reaction. Org Lett 4:4399–4401
Kubela, R.: US Patent 5, 908, 959, Process for the production of amino-1-hydroxy butylidene-1,1-bisphosphonic acid or salts thereof
Harris JM (1992) Poly (ethylene glycol), chemistry biotechnical and biomedical applications. Springer, New York, p 15
Herman S, Hooftman G, Schacht E (1995) Poly(ethylene glycol) with reactive end groups in modification of proteins. J Bioact Compat Polym 10:145–178
Soni J, Sahiba N, Sethiya A, Agarwal S (2020) Polyethylene glycol: a promising approach for sustainable organic synthesis. J Mol Liq 315:113766–113796
Israelachvili J (1997) The different faces of poly(ethylene glycol). Proc Natl Acad Sci USA 94:8378–8379
Mokhtari J, Azarnoosh S, Karimian K (2017) Resolution of racemic mixtures by phase transition of pegylated resolving agents. ACS Omega 2:8717–8722
Mokhtari J, Nourisefat M, Zamiri B, Fotouhi L, Zarnani A-H, Moosavi-Movahedi AA, Karimian K (2021) Novel method for the isolation of proteins and small target molecules from biological and aqueous media by salt-assisted phase transformation of their pegylated recognition counterparts. ACS Omega 6:7585–7597
Mase N, Nakai Y, Ohara N, Yoda H, Takabe K, Tanaka F, Barbas CFIII (2006) Organocatalytic direct asymmetric aldol reactions in water. J Am Chem Soc 128:734–735
Hayashi Y, Aratake S, Okano T, Takahashi J, Sumiya T, Shoji M (2006) Combined proline–surfactant organocatalyst for the highly diastereo- and enantioselective aqueous direct cross-aldol reaction of aldehydes. Angew Chem Int Ed 45:5527–5529
Romney DK, Arnold FH, Lipshutz BH (2018) Chemistry takes a bath: reactions in aqueous media. J Org Chem 83:7319–7322
Hayashi Y, Sumiya T, Takahashi J, Gotoh H, Urushima T, Shoji T (2006) Highly diastereo- and enantioselective direct aldol reactions in water. Angew Chem Int Ed 45:958–996
Scheilman JA (1997) Temperature, stability, and the hydrophobic interaction. Biophys J 73:2960–2964
Puglisi A, Rossi S (2021) Stereoselective organocatalysis and flow chemistry. Phys Sci Rev. https://doi.org/10.1515/psr-2018-0099
Bruckmann A, Krebs A, Bolm C (2008) Organocatalytic reactions: effects of ball milling, microwave and ultrasound irradiation. Green Chem 10:1131–1141
Hagen J (2015) Industrial catalysis, 3rd edn. Wiley-VCH Verlag GmbH & Co., Weinheim, pp 501–530
Leadbeater NE (2014) Microwave heating as a tool for organic chemistry in comprehensive organic synthesis. Enabl Technol Org Synth 9:234–286
Russo A, Leadbeater NE, Lattanzi A (2010) Convenient methodology for nitro-Michael addition of carbonyl compounds catalyzed by l-Proline using microwave heating. Lett Org Chem 7:98–102
Oakenful D, Fenwick DE (1977) Thermodynamics and mechanism of hydrophobic interaction. Aust J Chem 30:741–752
Sun Q, Fu Y, Wang W (2022) Temperature effects on hydrophobic interactions: implications for protein unfolding. Chem Phys 559:111550
Kondel P, Horak J, Tesarova J (1967) Variation of local void fraction in random and packed beds of equilateral cylinders. Ind Eng Chem Process Des Dev 7:250–252
Carberry JJ (1987) Physico-chemical aspects of mass and heat transfer in heterogeneous catalysis. In: Anderson JR et al (eds) Catalysis. Springer Verlag, Berlin, pp 131–171
Joshi SS, Ranade VV (2016) Industrial catalytic processes for fine and specialty chemicals. Elsevier Publications, Cambridge
Hosseini M, Stiasni N, Barbieri V, Kappe CO (2007) Microwave-assisted asymmetric organocatalysis. A probe for nonthermal microwave effects and the concept of simultaneous cooling. J Org Chem 72:1417–1424
Odedra A, Seeberger PH (2009) 5-(Pyrrolidin-2-yl)tetrazole-catalyzed aldol and Mannich reactions: acceleration and lower catalyst loading in a continuous-flow reactor. Angew Chem Int Ed 48:2699–2702
Szcześniak P, Staszewska-Krajewska O, Furman B, Mlynarski J (2017) Solid supported Hayashi-Jørgensen catalyst as an efficient and recyclable organocatalyst for asymmetric Michael addition reactions. Tetrahedron Asymmetry 28:1765–1773
Zcześniak P, Buda S, Lefevre L, Staszewska-Krajewska O, Mlynarski J (2019) Total asymmetric synthesis of (+)-paroxetine and (+)-femoxetine. Eur J Org Chem 2019:6973–6982
Oliveira PH, Santos BM, Leão RA, Miranda LS, San Gil RA, Souza RO, Finelliet FG (2019) From immobilization to catalyst use: a complete continuous-flow approach towards the use of immobilized organocatalysts. Chem Cat Chem 11:5553–5561
Zhou Y, Shan Z (2006) Chiral Diols: a new class of additives for direct aldol reaction catalyzed by l-proline. J Org Chem 71:9510–9512
Sun LHW, Yang D, Li G, Wang R (2016) Additive effects on asymmetric catalysis. Chem Rev 116:4006–4123
Pihko PM, Laurikainen KM, Usano A, Nyberg AI, Kaavi JA (2006) Effect of additives on the proline-catalyzed ketone-aldehyde aldol reactions. Tetrahedron 62:317–328
Notz W, Tanaka F, Barbas CF (2004) Development of direct catalytic asymmetric aldol, Mannich, Michael, and Diels-Alder reactions. Acc Chem Res 37:580–591
Zotova N, Franzke A, Armstrong A, Blackmond DG (2007) Clarification of the role of water in proline-mediated aldol reactions. J Am Chem Soc 129:15100–15101
Tam JWO, Klotz IM (1973) Protonation of amides by triflouoroacetic acid: infrared and nuclear magnetic resonance studies. Spectrochim Acta 29A:633–644
Zotova N, Broadbelt LJ, Armstrong A, Blackmond DG (2009) Kinetic and mechanistic studies of proline-mediated direct intermolecular aldol reactions. Bioorg Med Chem Lett 19:3934–3937
Sunoj RB (2016) Transition state models for understanding the origin of chiral induction in asymmetric catalysis. Acc Chem Res 49:1019–1028
Song CE, Park SJ, Hwang I-S, Jung MJ, Shim SY, Bae HY, Jung JY (2019) Hydrophobic chirality amplification in confined water cages. Nat Comm 10:851
Varghese JJ, Mushrif SH (2019) Origins of complex solvent effects on chemical reactivity and computational tools to investigate them: a review. React Chem Eng 4:165–206
Fang Y-R, MacMillar S, Eriksson J, Kołodziejska-Huben M, Dybała-Defratyka A, Paneth P, Matsson O, Westaway KC (2006) The effect of solvent on the structure of the transition state for the SN2 reaction between cyanide ion and ethyl chloride in DMSO and THF probed with six different kinetic isotope effects. J Org Chem 71:4742–4747
Morris W, Lorance ED, Gould IR (2019) Understanding the solvent contribution to chemical reaction barriers. J Phys Chem A 123:10490–10499
Bahmanyar S, Houk KN (2001) Transition states of amine-catalyzed aldol reactions involving enamine intermediates: theoretical studies of mechanism, reactivity, and stereoselectivity. J Am Chem Soc 123:11273–11283
Karnik A, Hasan M (2021) Stereochemistry: a three-dimensional insight. Elsevier, Amsterdam
https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/sterslct.htm
Sunoj RB (2011) Proline-derived organocatalysis and synergism between theory and experiments. Comput Mol Sci 1:920–931
Perrin CL, Chang K-L (2016) The complete mechanism of an aldol condensation. J Org Chem 81:631–635
Marche C, Ferronato C, Jose J (2003) Solubilities of n-alkanes (C6 to C8) in Water from 30 to 180 °C. J Chem Eng Data 48:967–997
Bogunia M, Makowski M (2020) Influence of ionic strength on hydrophobic interactions in water: dependence on solute size and shape. J Phys Chem B 124:10326–10336
Jiang M, Zhu S-F, Yang Y, Gong L-Z, Zhou X-G, Zhou Q-L (2006) Asymmetric aldol reactions catalyzed by new spiro diamine derivatives. Tetrahedron Asymmetry 17:384–387
Hayashi Y, Aratake S, Itoh T, Okano T, Sumiya T, Shoji M (2007) Dry and wet prolines for asymmetric organic solvent-free aldehyde and aldehyde–ketone aldol reactions. Chem Commun. https://doi.org/10.1039/B613262F
Sekiguchi Y, Sasaoka A, Shimomoto A, Fujioka S, Kotsuki H (2003) High-pressure-promoted asymmetric aldol reactions of ketones with aldehydes catalyzed by l-proline. Synlett. https://doi.org/10.1055/s-2003-41424
Shajahan R, Sarang R, Saithalavi A (2022) Polymer supported proline-based organocatalysts in asymmetric aldol reactions: a review. Curr Organocatal 9:124–146
Guillena G, Alonso D, Baeza A, Chinchilla R, Flores-Ferrándiz J, Gómez-Martínez M, Trillo P (2015) Pursuing chemical efficiency by using supported organocatalysts for asymmetric reactions under aqueous conditions. Curr Organocatal 2:102–123
Nweke, M.C.: Chromatography resin characterization to analyze lifetime and performance during biopharmaceutical manufacture. A thesis submitted for the degree of Doctor of Philosophy, Department of Biochemical Engineering University College London (2017)
Author information
Authors and Affiliations
Contributions
All authors contributed to this study. The concept was developed by KK and the work was supervised by JM and carried out by FH-D, HZ and AY.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hosseini-Dastjerdi, F., Zandieh, H., Yari, A. et al. N-PEGylated (L)-Prolinamide: A Homogeneous, Solvent-Free, and Recyclable Catalyst for Scalable Enantioselective Aldol Reaction. Catal Lett (2024). https://doi.org/10.1007/s10562-024-04620-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10562-024-04620-2