Abstract
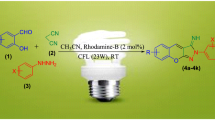
Herein, an apposite and straightforward methodology has been established for the synthesis of 4,5-dihydro-1H-pyrazole and its derivatives by using Chalcones and Hydrazines as substrates in EtOH under 20W CFL irradiation at room temperature. The distinctive trait of this protocol is the utilization of an efficient, green, highly water soluble organophotoredox catalyst “Rhodamine B”, a simple red xanthene dye, to create C–N bonds via formation of free radicals and eventually terminating with intramolecular cyclization. Short reaction time, environmentally benign approach, cost effectiveness, feasibility and adaptability with respect to a wide range of substrates and good to excellent yield of the product supplements the synthesis.
Graphical Abstract






Similar content being viewed by others
References
Kulshreshtha A (2015) Soc Issues Environ Probl 3:1
Ivankovic A, Dronjic A, Bevanda AM, Talic S (2017) IJSGE 6:39
Lang X, Zhaob J, Xiaodong C (2016) Chem Soc Rev 45:3026–30386
Xu G, Xu P (2021) Chem Commun 57:12914–12935
Chen J, Hu X, Lu L, Xiao W (2016) Chem Soc Rev 45:2044–2056
Dai C, Liu B (2020) Energy Environ Sci 13:24–52
Uygur M, Danelzik T, Mancheño OG (2019) Chem Commun 55:2980–2983
Srivastava V, Singh PP (2017) RSC Adv 7:31377–31392
Peng Y, Feng CT, Li YQ, Chen FX, Xu K (2019) Org Biomol Chem 17:6570–6573
Saravanan R, Gupta VK, Narayanan V, Stephen A (2014) J Taiwan Inst Chem Eng 45:1910–1917
Helm MPV, Klemm B, Eelkema R (2019) Nat Rev Chem 3:491–508
Zhang JJ, Cheng YB, Duan XH (2017) Chin J Chem 35:311–315
Xie LY, Hu JL, Song YX, Jia GK, Lin YW, He JY, Cao Z, He WM (2019) ACS Sustain Chem Eng 7:19993–19999
Xie LY, Chen YL, Qin L, Wen Y, Xie JW, Tan JX, Huang Y, Cao Z, He WM (2019) Org Chem Front 6:3950–3955
He H, Xu N, Zhang H, Chen B, Hu Z, Guo K, Chun J, Cao S, Zhu Y (2021) RSC Adv 11:17340–17345
Aboul-Enein MN, El-Azzouny AA, Attia MI, Maklad YA, Amin KM, Abdel-Rehim M, El Behairy MF (2012) J Med Chem 47:360–369
Li QS, Lv XH, Zhang YB, Dong JJ, Zhou WP, Yang Y, Zhu HL (2012) Bioorg Med Chem Lett 22:6596–6601
Goodell JR, Puig-Basagoiti F, Forshey BM, Shi PY, Ferguson DM (2006) J Med Chem 49:2127–2137
Agrawal M, Sonar PK, Saraf SK (2011) Med Chem Res 21:3376–3381
Sivakumar PM, Seenivasan SP, Kumar V, Doble M (2010) Chem Lett 20:3169–3172
Bhat P, Shridhar G, Ladage S, Ravishankar L (2017) J Chem Sci 129:1441–1448
Gadekar SP, Pawar GT, Magar RR, Lande MK (2020) Polycycl Aromat Compd 40:126–134
Pise AS, Jadhav SD, Burungale AS, Devkate SS, Gawade RB (2018) Asian J Chem 30:894–896
Babiola AS, Pothiappan V, Subburethinam R (2018) RSC Adv 8:30071–30075
Jaiswal D, Tiwari J, Singh S, Sharma AK, Singh J, Singh J (2018) Curr Organocatalysis 5:229–238
Mishra A, Srivastava M, Rai P, Yadav S, Tripathi BP, Singh J, Singh J (2016) RSC Adv 6:49164–49172
Mishra A, Jaiswal A, Sharma AK, Pandey YK, Singh J, Singh J (2023). Catal Lett. https://doi.org/10.1007/s10562-023-04279-1
Mishra A, Rai P, Singh J, Singh J (2018) ChemistrySelect 3:8408–8414
Mishra A, Rai P, Srivastava M, Tripathi BP, Yadav S, Singh J, Singh J (2017) Catal Lett 147:2600–2611
Tripathi BP, Mishra A, Rai P, Pandey YK, Srivastava M, Yadav S, Singh J, Singh J (2017) New J Chem 41:11148–11154
Sharma AK, Jaiswal A, Mishra A, Jaiswal D, Singh S, Singh J, Singh J (2020) New J Chem 44:13350–13356
Yadav AK, Yadav LDS (2015) Org Biomol Chem 13:2606–2611
Bu MJ, Lu GP, Cai C (2016) Catal Sci Technol 6:413–416
Acknowledgements
The authors are thankful to SAIF, Punjab University, Chandigarh, India and SAIF, CDRI, Lucknow, India for providing spectral data. Prof. Jagdamba Singh acknowledges the financial support from UGC, New Delhi in the form of BSR Faculty Fellowship (No. F.18-1/2011 (BSR)).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mishra, A., Pandey, Y.K., Sharma, A.K. et al. A Rhodamine-B Catalyzed Visible-Light-Mediated Benign Synthetic Route for 4,5-Dihydro-1H-pyrazoles. Catal Lett (2024). https://doi.org/10.1007/s10562-024-04591-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10562-024-04591-4