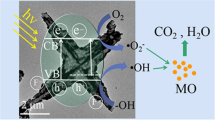
Abstract
This study investigates the impact of calcination temperature and the use of monochloroacetic acid (MCAA) as a complexing agent during the synthesis of TiO2 heterojunctions on the photocatalytic performance of TiO2 containing a heterojunction of anatase (A) and brookite (B). The TiO2 samples were dried at 100 °C and subjected to calcination in the temperature range of 200–500 °C. As the calcination temperature increased, changes in chemical composition, phase composition, specific surface area, pore size distribution, and band gap energy were observed, influencing the photocatalytic behavior during Acid Orange (AO7) photodegradation. With increasing calcination temperature, the anatase content slightly increased to 81.8 wt.%, while the brookite content decreased. Additionally, the anatase crystallite size increased from 5 to 7 nm, and the brookite crystallite size increased from 8 to 11 nm. These phase changes were characterized by a slight decrease in the brookite/anatase (B/A) molar ratio from 0.27 to 0.22. Concurrently, the specific surface area of TiO2 samples decreased from 180 to 70 m2 g−1, accompanied by alterations in the pore size distribution. The decrease in photocatalytic activity was evident, as the apparent rate constant kapp of AO7 photodegradation declined from 2.1 × 10–3 min−1 (100 °C) to 0.7 × 10–3 min−1 (500 °C). This reduction was attributed to the partial destruction of anatase/brookite heterojunctions on the photocatalyst surface, which underwent changes in size and surface structure due to sintering. Moreover, the presence of Ti3+, Ti4+, Ti–O, and Ti–OH hydroxyl groups played a significant role in the degradation process. Overall, this investigation sheds light on the crucial role of calcination temperature in modulating the photocatalytic properties of TiO2 heterojunctions and highlights the importance of heterojunction in enhancing photocatalytic activity.
Graphical Abstract



















Similar content being viewed by others
Change history
14 December 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10562-023-04531-8
References
Ajmal A, Majeed I, Malik RN et al (2014) Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: a comparative overview. RSC Adv 4:37003–37026. https://doi.org/10.1039/C4RA06658H
Lim TT, Yap PS, Srinivasan M, Fane AG (2011) TiO2/AC composites for synergistic adsorption-photocatalysis processes: present challenges and further developments for water treatment and reclamation. Crit Rev Environ Sci Technol 41:1173–1230. https://doi.org/10.1080/10643380903488664
Shayegan Z, Lee CS, Haghighat F (2018) TiO2 photocatalyst for removal of volatile organic compounds in gas phase—a review. Chem Eng J 334:2408–2439. https://doi.org/10.1016/J.CEJ.2017.09.153
Drobná H, Dubnová L, Rokici A (2018) Nd/TiO2 anatase–brookite photocatalysts for photocatalytic decomposition of methanol. Front Chem 6:1–11. https://doi.org/10.3389/fchem.2018.00044
Mustapha S, Ndamitso MM, Abdulkareem AS et al (2020) Application of TiO2 and ZnO nanoparticles immobilized on clay in wastewater treatment: a review. Appl Water Sci. https://doi.org/10.1007/s13201-019-1138-y
Singh R, Dutta S (2018) Synthesis and characterization of solar photoactive TiO2 nanoparticles with enhanced structural and optical properties. Adv Powder Technol 29:211–219. https://doi.org/10.1016/j.apt.2017.11.005
Košević M, Šekularac G, Živković L et al (2017) TiO2 from colloidal dispersion as support in Pt/TiO2 nanocomposite for electrochemical applications. Croat Chem Acta 90:251–258. https://doi.org/10.5562/cca3175
D’Elia D, Beauger C, Hochepied JF et al (2011) Impact of three different TiO2 morphologies on hydrogen evolution by methanol assisted water splitting: nanoparticles, nanotubes and aerogels. Int J Hydrogen Energy 36:14360–14373. https://doi.org/10.1016/j.ijhydene.2011.08.007
Addawiyah E, Gunlazuardi J (2018) Nickel sulfide sensitized TiO2 nanotubes system as photo-anode: will this system active under visible light and why? AIP Conf Proc. https://doi.org/10.1063/1.5064087
Mahy JG, Cerfontaine V, Poelman D et al (2018) Highly efficient low-temperature N-doped TiO2 catalysts for visible light photocatalytic applications. Materials (Basel) 11:1–20. https://doi.org/10.3390/ma11040584
Huang L, Li R, Chong R et al (2014) Cl-making overall water splitting possible on TiO2-based photocatalysts. Catal Sci Technol 4:2913–2918. https://doi.org/10.1039/c4cy00408f
Huang G, Zhang J, Jiang F et al (2020) Excellent photoelectrochemical activity of Bi2S3 nanorod/TiO2 nanoplate composites with dominant 001 facets. J Solid State Chem 281:121041. https://doi.org/10.1016/J.JSSC.2019.121041
Zeshan M, Bhatti IA, Mohsin M et al (2022) Remediation of pesticides using TiO2 based photocatalytic strategies: a review. Chemosphere 300:134525. https://doi.org/10.1016/j.chemosphere.2022.134525
Nur ASM, Sultana M, Mondal A et al (2022) A review on the development of elemental and codoped TiO2 photocatalysts for enhanced dye degradation under UV–vis irradiation. J Water Process Eng 47:2214–7144. https://doi.org/10.1016/j.jwpe.2022.102728
Karabelas AJ, Plakas KV, Sarasidis VC, Patsios SI (2013) Novel advanced oxidation process systems for water purification-progress and prospects for large scale applications
Dharma HNC, Jaafar J, Widiastuti N et al (2022) A review of titanium dioxide (TiO2)-based photocatalyst for oilfield-produced water treatment. Membranes 12:345. https://doi.org/10.3390/MEMBRANES12030345
Chen Y, Dong X, Cao Y et al (2017) Enhanced photocatalytic activities of low-bandgap TiO2-reduced graphene oxide nanocomposites. J Nanopart Res 19:1–13. https://doi.org/10.1007/s11051-017-3871-1
Zaleska-Medynska A, Grabowska E, Marchelek M et al (2018) Metal oxide photocatalysts. Met Oxide-Based Photocatal. https://doi.org/10.1016/B978-0-12-811634-0.00003-2
Puskelova J, Baia L, Vulpoi A et al (2014) Photocatalytic hydrogen production using TiO2–Pt aerogels. Chem Eng J 242:96–101. https://doi.org/10.1016/j.cej.2013.12.018
Hao YN, Chen T, Zhang X et al (2019) Ti–Ti σ bond at oxygen vacancy inducing the deep defect level in anatase TiO2 (101) surface. J Chem Phys. https://doi.org/10.1063/15108595
Bharti B, Kumar S, Lee HN, Kumar R (2016) Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci Rep. https://doi.org/10.1038/srep32355
Lettieri S, Pavone M, Fioravanti A et al (2021) Charge carrier processes and optical properties in TiO2 and TiO2-based heterojunction photocatalysts: a review. Materials 14:1645. https://doi.org/10.3390/MA14071645
Shahiduzzaman M, Visal S, Kuniyoshi M et al (2018) Low-temperature-processed brookite-based TiO2 heterophase junction enhances performance of planar perovskite solar cells. Nano Lett. https://doi.org/10.1021/acs.nanolett.8b04744
Bhave R (2007) Synthesis and photocatalysis study of brookite phase titanium dioxide nanoparticles, pp 1–73
Hu Y, Tsai HL, Huang CL (2003) Effect of brookite phase on the anatase–rutile transition in titania nanoparticles. J Eur Ceram Soc 23:691–696. https://doi.org/10.1016/S0955-2219(02)00194-2
Hanaor DAH, Sorrell CC (2011) Review of the anatase to rutile phase transformation. J Mater Sci 46:855–874. https://doi.org/10.1007/s10853-010-5113-0
Koparde VN, Cummings PT (2008) Phase transformations during sintering of titania nanoparticles. ACS Nano 2:1620–1624. https://doi.org/10.1021/nn800092m
Kandiel TA, Feldhoff A, Robben L et al (2010) Tailored titanium dioxide nanomaterials: anatase nanoparticles and brookite nanorods as highly active photocatalysts. Chem Mater 22:2050–2060. https://doi.org/10.1021/cm903472p
Wang Z, Wang Y, Zhang W et al (2019) Fabrication of TiO2 (B)/anatase heterophase junctions at high temperature via stabilizing the surface of TiO2 (B) for enhanced photocatalytic activity. J Phys Chem C. https://doi.org/10.1021/acs.jpcc.8b09763
Yu X, Kim B, Kim YK (2013) Highly enhanced photoactivity of anatase TiO2 nanocrystals by controlled hydrogenation-induced surface defects. ACS Catal 3:2479–2486. https://doi.org/10.1021/CS4005776/SUPPL_FILE/CS4005776_SI_001.PDF
Tian G, Fu H, Jing L, Tian C (2009) Synthesis and photocatalytic activity of stable nanocrystalline TiO2 with high crystallinity and large surface area. J Hazard Mater 161:1122–1130. https://doi.org/10.1016/J.JHAZMAT.2008.04.065
Hurum DC, Agrios AG, Gray KA et al (2003) Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 using EPR. J Phys Chem B 107:4545–4549. https://doi.org/10.1021/jp0273934
Huang C, Guo R, Pan W et al (2019) One-dimension TiO2 nanostructures with enhanced activity for CO2 photocatalytic reduction. Appl Surf Sci 464:534–543. https://doi.org/10.1016/J.APSUSC.2018.09.114
Reza Gholipour M, Dinh CT, Béland F, Do TO (2015) Nanocomposite heterojunctions as sunlight-driven photocatalysts for hydrogen production from water splitting. Nanoscale 7:8187–8208. https://doi.org/10.1039/c4nr07224c
Wang X, Li C, Shi Z et al (2018) The investigation of an organic acid assisted sol–gel method for preparing monolithic zirconia aerogels. RSC Adv 8:8011–8020. https://doi.org/10.1039/c7ra13041d
Khan H, Berk D (2014) Effect of a chelating agent on the physicochemical properties of TiO2: characterization and photocatalytic activity. Catal Lett 144:890–904. https://doi.org/10.1007/s10562-014-1233-5
Mahmoud HA, Narasimharao K, Ali TT, Khalil KMS (2018) Acidic peptizing agent effect on anatase-rutile ratio and photocatalytic performance of TiO2 nanoparticles. Nanoscale Res Lett. https://doi.org/10.1186/s11671-018-2465-x
Zhang Y, Hawboldt K, Zhang L et al (2022) Carbonaceous nanomaterial-TiO2 heterojunctions for visible-light-driven photocatalytic degradation of aqueous organic pollutants. Appl Catal A Gen 630:118460. https://doi.org/10.1016/J.APCATA.2021.118460
Li J, Liu B, Han X et al (2021) Direct Z-scheme TiO2−x/AgI heterojunctions for highly efficient photocatalytic degradation of organic contaminants and inactivation of pathogens. Sep Purif Technol 261:118306. https://doi.org/10.1016/J.SEPPUR.2021.118306
Parul KK, Badru R et al (2020) Photodegradation of organic pollutants using heterojunctions: a review. J Environ Chem Eng 8:103666. https://doi.org/10.1016/J.JECE.2020.103666
Guohui T, Honggang F, Liqiang J et al (2008) Preparation and characterization of stable biphase TiO2 photocatalyst with high crystallinity, large surface area, and enhanced photoactivity. J Phys Chem C 112:3083–3089. https://doi.org/10.1021/jp710283p
Jbeli A, Ferraria AM, Botelho do Rego AM et al (2018) Hybrid chitosan-TiO2/ZnS prepared under mild conditions with visible-light driven photocatalytic activity. Int J Biol Macromol 116:1098–1104. https://doi.org/10.1016/J.IJBIOMAC.2018.05.141
Jiang G, Huang Q, Kenarsari SD et al (2015) A new mesoporous amine-TiO2 based pre-combustion CO2 capture technology. Appl Energy 147:214–223. https://doi.org/10.1016/j.apenergy.2015.01.081
Shi L, Wang T, Zhang H et al (2015) An amine-functionalized iron(III) metal-organic framework as efficient visible-light photocatalyst for Cr(VI) reduction. Adv Sci 2:1500006. https://doi.org/10.1002/advs.201500006
Ardizzone S, Bianchi CL, Cappelletti G et al (2007) Tailored anatase/brookite nanocrystalline TiO2. The optimal particle features for liquidand gas-phase photocatalytic reactions. J Phys Chem C 111:13222–13231. https://doi.org/10.1021/JP0741096/ASSET/IMAGES/MEDIUM/JP0741096E00003.GIF
Gupta SM, Tripathi M (2011) A review of TiO2 nanoparticles. Chin Sci Bull 56:1639–1657. https://doi.org/10.1007/s11434-011-4476-1
Cihlar J, Kasparek V, Kralova M, Castkova K (2015) Biphasic anatase–brookite nanoparticles prepared by sol–gel complex synthesis and their photocatalytic activity in hydrogen production. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2015.01.008
Cihlar J, Tinoco Navarro LK, Kasparek V et al (2021) Influence of LA/Ti molar ratio on the complex synthesis of anatase/brookite nanoparticles and their hydrogen production. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2020.12.080
He F, Ma F, Li J et al (2014) Effect of calcination temperature on the structural properties and photocatalytic activities of solvothermal synthesized TiO2 hollow nanoparticles. Ceram Int 40:6441–6446. https://doi.org/10.1016/J.CERAMINT.2013.11.094
Muthee DK, Dejene BF (2021) Effect of annealing temperature on structural, optical, and photocatalytic properties of titanium dioxide nanoparticles. Heliyon 7:e07269. https://doi.org/10.1016/J.HELIYON.2021.E07269
Sugapriya S, Sriram R, Lakshmi S (2013) Effect of annealing on TiO2 nanoparticles. Optik (Stuttg) 124:4971–4975. https://doi.org/10.1016/J.IJLEO.2013.03.040
Heng I, Lai CW, Juan JC et al (2019) Low-temperature synthesis of TiO2 nanocrystals for high performance electrochemical supercapacitors. Ceram Int 45:4990–5000. https://doi.org/10.1016/j.ceramint.2018.11.199
Yu J, Yu H, Cheng B, Trapalis C (2006) Effects of calcination temperature on the microstructures and photocatalytic activity of titanate nanotubes. J Mol Catal A Chem 249:135–142. https://doi.org/10.1016/J.MOLCATA.2006.01.003
Zeng Y, Zhang S, Wang Y et al (2017) The effects of calcination atmosphere on the catalytic performance of Ce-doped TiO2 catalysts for selective catalytic reduction of NO with NH3. RSC Adv 7:23348–23354. https://doi.org/10.1039/c7ra03166a
Lal M, Sharma P, Ram C (2021) Calcination temperature effect on titanium oxide (TiO2) nanoparticles synthesis. Optik (Stuttg) 241:166934. https://doi.org/10.1016/J.IJLEO.2021.166934
Kim MG, Kang JM, Lee JE et al (2021) Effects of calcination temperature on the phase composition, photocatalytic degradation, and virucidal activities of TiO2 nanoparticles. ACS Omega 6:10668–10678. https://doi.org/10.1021/ACSOMEGA.1C00043/ASSET/IMAGES/LARGE/AO1C00043_0009.JPEG
Phromma S, Wutikhun T, Kasamechonchung P et al (2020) Effect of calcination temperature on photocatalytic activity of synthesized TiO2 nanoparticles via wet ball milling sol–gel method. Appl Sci 10:993. https://doi.org/10.3390/APP10030993
Wahab AK, Ould-Chikh S, Meyer K, Idriss H (2017) On the “possible” synergism of the different phases of TiO2 in photo-catalysis for hydrogen production. J Catal 352:657–671. https://doi.org/10.1016/J.JCAT.2017.04.033
Cihlar J, Cihlar J, Bartonickova E (2013) Low-temperature sol–gel synthesis of anatase nanoparticles modified by Au, Pd and Pt and activity of TiO2/Au, Pd, Pt photocatalysts in water splitting. J Sol-Gel Sci Technol. https://doi.org/10.1007/s10971-012-2955-8
Luís AM, Neves MC, Mendonça MH, Monteiro OC (2011) Influence of calcination parameters on the TiO2 photocatalytic properties. Mater Chem Phys 125:20–25. https://doi.org/10.1016/J.MATCHEMPHYS.2010.08.019
Zhang Q, Gao L, Guo J (2000) Effects of calcination on the photocatalytic properties of nanosized TiO2 powders prepared by TiCl4 hydrolysis. Appl Catal B Environ 26:207–215. https://doi.org/10.1016/S0926-3373(00)00122-3
Saikumari N, Dev SM, Dev SA (2021) Effect of calcination temperature on the properties and applications of bio extract mediated titania nano particles. Sci Rep 111(11):1–17. https://doi.org/10.1038/s41598-021-80997-z
Doeuff S, Henry M, Sanchez C, Livage J (1987) Hydrolysis of titanium alkoxides: modification of the molecular precursor by acetic acid. J Non Cryst Solids 89:206–216. https://doi.org/10.1016/S0022-3093(87)80333-2
Hao R, Jiang B, Li M et al (2015) Fabrication of mixed-crystalline-phase spindle-like TiO2 for enhanced photocatalytic hydrogen production. Sci China Mater 58:363–369. https://doi.org/10.1007/s40843-015-0052-3
Rouquerol J, Rouquerol F, Llewellyn P, Maurin G, Sing KSW (2014) Adsorption by powders and porous solids: principles, methodology and applications. Elsevier, Amsterdam
Zhang A, Zhang Z, Chen J et al (2015) Effect of calcination temperature on the activity and structure of MnOx/TiO2 adsorbent for Hg0 removal. Fuel Process Technol 135:25–33. https://doi.org/10.1016/J.FUPROC.2014.10.007
Bharti B, Kumar S, Lee HN, Kumar R (2016) Formation of oxygen vacancies and Ti3+ state in TiO2 thin film and enhanced optical properties by air plasma treatment. Sci Rep 6:1–12. https://doi.org/10.1038/srep32355
Kar P, Aggarwal D, Shukla K, Gupta RK (2022) Defect state modulation of TiO2 nanostructures for photocatalytic abatement of emerging pharmaceutical pollutant in wastewater effluent. Adv Energy Sustain Res 3:2100162. https://doi.org/10.1002/AESR.202100162
Chattopadhyay S, Maiti S, Das I et al (2016) Electrospun TiO2–rGO composite nanofibers with ordered mesopores by molecular level assembly: a high performance anode material for lithium-ion batteries. Adv Mater Interfaces 3:1600761. https://doi.org/10.1002/ADMI.201600761
Wang WS, Wang DH, Qu WG et al (2012) Large ultrathin anatase TiO2 nanosheets with exposed 001 facets on graphene for enhanced visible light photocatalytic activity. J Phys Chem C 116:19893–19901. https://doi.org/10.1021/JP306498B/SUPPL_FILE/JP306498B_SI_001.PDF
Elmouwahidi A, Bailón-García E, Castelo-Quibén J et al (2018) Carbon–TiO2 composites as high-performance supercapacitor electrodes: synergistic effect between carbon and metal oxide phases. J Mater Chem A 6:633–644. https://doi.org/10.1039/C7TA08023A
Chen C, Liu X, Long H et al (2019) Preparation and photocatalytic performance of graphene oxide/WO3 quantum dots/TiO2@SiO2 microspheres. Vacuum 164:66–71. https://doi.org/10.1016/J.VACUUM.2019.03.002
Qiu C, Lin J, Shen J et al (2020) Regulation of the rutile/anatase TiO2 heterophase interface by Ni12P5 to improve photocatalytic hydrogen evolution. Catal Sci Technol 10:3709–3719. https://doi.org/10.1039/d0cy00634c
Cihlar J, Navarro LKT, Cihlar J et al (2022) Influence of substituted acetic acids on “bridge” synthesis of highly photocatalytic active heterophase TiO2 in hydrogen production. J Sol-Gel Sci Technol. https://doi.org/10.1007/S10971-022-06011-8/FIGURES/14
Mao Q, Ren Y, Luo KH, Li S (2015) Sintering-induced phase transformation of nanoparticles: a molecular dynamics study. J Phys Chem C 119:28631–28639. https://doi.org/10.1021/acs.jpcc.5b08625
Yu JG, Yu HG, Cheng B et al (2003) The effect of calcination temperature on the surface microstructure and photocatalytic activity of TiO2 thin films prepared by liquid phase deposition. J Phys Chem B 107:13871–13879. https://doi.org/10.1021/jp036158y
Asenjo NG, Santamaría R, Blanco C et al (2013) Correct use of the Langmuir–Hinshelwood equation for proving the absence of a synergy effect in the photocatalytic degradation of phenol on a suspended mixture of titania and activated carbon. Carbon N Y 55:62–69. https://doi.org/10.1016/J.CARBON.2012.12.010
Qourzal S, Barka N, Tamimi M et al (2008) Photodegradation of 2-naphthol in water by artificial light illumination using TiO2 photocatalyst: identification of intermediates and the reaction pathway. Appl Catal A Gen 334:386–393. https://doi.org/10.1016/J.APCATA.2007.09.034
Khan MM, Ansari SA, Pradhan D et al (2014) Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J Mater Chem A 2:637–644. https://doi.org/10.1039/c3ta14052k
Liu Y, Chen X, Li J, Burda C (2005) Photocatalytic degradation of azo dyes by nitrogen-doped TiO2 nanocatalysts. Chemosphere 61:11–18. https://doi.org/10.1016/J.CHEMOSPHERE.2005.03.069
Zhang X, Sun DD, Li G, Wang Y (2008) Investigation of the roles of active oxygen species in photodegradation of azo dye AO7 in TiO2 photocatalysis illuminated by microwave electrodeless lamp. J Photochem Photobiol A Chem 199:311–315. https://doi.org/10.1016/J.JPHOTOCHEM.2008.06.009
Chen YW, Hsu YH (2021) Effects of reaction temperature on the photocatalytic activity of TiO2 with Pd and Cu cocatalysts. Catalyst 11:966. https://doi.org/10.3390/CATAL11080966
Fischer K, Gawel A, Rosen D et al (2017) Low-temperature synthesis of anatase/rutile/brookite TiO2 nanoparticles on a polymer membrane for photocatalysis. Catalysts. https://doi.org/10.3390/catal7070209
Leyva-Porras C, Toxqui-Teran A, Vega-Becerra O et al (2015) Low-temperature synthesis and characterization of anatase TiO2 nanoparticles by an acid assisted sol-gel method. J Alloys Compd. https://doi.org/10.1016/j.jallcom.2015.06.041
Stylidi M, Kondarides DI, Verykios XE (2003) Pathways of solar light-induced photocatalytic degradation of azo dyes in aqueous TiO2 suspensions. Appl Catal B Environ 40:271–286. https://doi.org/10.1016/S0926-3373(02)00163-7
Acknowledgements
The work was carried out in the frame of the COST Action CA18125 "Advanced Engineering and Research of aerogels for Environment and Life Sciences" (AERoGELS) and funded by the European Commission". The authors acknowledge the support of the Ministry of Education, Youth and Sports of the Czech Republic under Grant No. LTC20019. We also acknowledge CzechNanoLab Research Infrastructure supported by MEYS CR (LM2023051).
Funding
This work was supported by Ministry of Education, Youth and Sports of the Czech Republic under Grant numbers LTC20019. We also acknowledge CzechNanoLab Research Infrastructure supported by MEYS CR (LM2023051).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study as it follows. KTN: conceptualization, methodology, investigation, formal analysis writing—original draft, review & editing. JC: supervision, investigation, validation, formal analysis, writing—review & editing. KC: project administration, validation, writing—review & editing. JM: formal analysis, validation, writing—review & editing. JK: formal analysis, validation, writing—review & editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of the article was revised: The project number in the Funding and acknowledgment has been corrected.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tinoco Navarro, L.K., Cihlar, J., Michalicka, J. et al. Effect of MCAA Synthesis and Calcination Temperature on Heterojunction Formation and Photocatalytic Activity of Biphasic TiO2 (B/A). Catal Lett 154, 2422–2442 (2024). https://doi.org/10.1007/s10562-023-04489-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-023-04489-7