Abstract
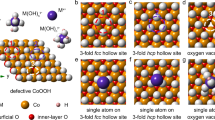
Anchoring noble metals to non-noble metals oxide to create single-atom catalysts (SACs) was an appealing approach to improve atomic efficiency and catalyst activity with simultaneous reduction of cost. However, knowledge of how single-atom anchors at unique loading positions and varying numbers of coordination atoms behavior in oxygen evolution reaction (OER) was still inadequate. Herein, the Ir was chosen to anchor at three-fold face center cubic (FCC) hollow sites, three-fold hexagonal close packed (HCP) hollow sites, and Oxygen Vacancy (OV) sites on Co3O4 (111) surface with different coordination environments by first-principles study of OER. The results displayed that the overpotential (η) of threefold FCC hollow sites with four coordination oxygens (FCC-4O) was 0.35 V, which was the lowest overpotential among our catalysts. The d-band center and Bader charge results demonstrated the coordination oxygen could change Ir electron structure, specifically FCC-4O, making the adsorption between the carrier and the intermediate more moderate for reducing the energy barrier of the potential-determining step. When Ir possesses five coordination oxygens, the HCP sites spontaneously transformed into the FCC sites, showing it was an unstable state. In addition, band gap studies indicated that the OV sites catalysts had a reduced band gap and stronger electron transport ability, notably for the Ir with four-coordination hydroxyl group (OV-4OH), which led to high OER performance (η = 0.46 V). Finally, the solvation effect and kinetic stability were tested, it could be found that both FCC-4O and OV-4OH showed good OER performance, especially FCC has good stability and may be more possible in experimental conditions.
Graphical Abstract
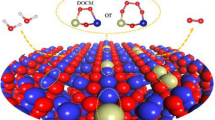
The figure described volcano diagram of our catalysts and the illustration of FCC sites, HCP sites, and OV sites. FCC-4O had the best OER performance among our catalysts with η = 0.35 V.











Similar content being viewed by others
References
Chu S, Majumdar A (2012) Opportunities and challenges for a sustainable energy future. Nature 488:294–303
Kibsgaard J, Chorkendorff I (2019) Considerations for the scaling-up of water splitting catalysts. Nat Energy 4:430–433
Suen NT, Hung SF, Quan Q, Zhang N, Xu YJ, Chen HM (2017) Electrocatalysis for the oxygen evolution reaction: recent development and future perspectives. Chem Soc Rev 46:337–365
Czelej K, Colmenares JC, Jabłczyńska K, Ćwieka K, Werner Ł, Gradoń L (2021) Sustainable hydrogen production by plasmonic thermophotocatalysis. Catal Today 380:156–186
Zhang B, Zheng X, Voznyy O, Comin R, Bajdich M, García-Melchor M, Han L, Xu J, Liu M, Zheng L, García de Arquer FP, Dinh CT, Fan F, Yuan M, Yassitepe E, Chen N, Regier T, Liu P, Li Y, De Luna P, Janmohamed A, Xin HL, Yang H, Vojvodic A, Sargent EH (2016) Homogeneously dispersed multimetal oxygen-evolving catalysts. Science 352:333–337
Montoya JH, Seitz LC, Chakthranont P, Vojvodic A, Jaramillo TF, Nørskov JK (2017) Materials for solar fuels and chemicals. Nat Mater 16:70–81
Zhu YP, Guo C, Zheng Y, Qiao S-Z (2017) Surface and interface engineering of noble-metal-free electrocatalysts for efficient energy conversion processes. Acc Chem Res 50:915–923
Yue C, Wang L, Wang H, Du J, Lei M, Pu M (2022) First-principles study on the electrocatalytic oxygen evolution reaction on the (110) surfaces of layered double hydroxides. J Phys Chem C 126:18351–18365
Seh ZW, Kibsgaard J, Dickens CF, Chorkendorff I, Nørskov JK, Jaramillo TF (2017) Combining theory and experiment in electrocatalysis: insights into materials design. Science 355:eaad4998
Song F, Bai L, Moysiadou A, Lee S, Hu C, Liardet L, Hu X (2018) Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: an application-inspired renaissance. J Am Chem Soc 140:7748–7759
Man IC, Su HY, Calle-Vallejo F, Hansen HA, Martinez JI, Inoglu NG, Kitchin J, Jaramillo TF, Norskov JK, Rossmeisl J (2011) Universality in oxygen evolution electrocatalysis on oxide surfaces. ChemCatChem 3:1159–1165
Fang Y-H, Liu Z-P (2010) Mechanism and Tafel lines of electro-oxidation of water to oxygen on RuO2(110). J Am Chem Soc 132:18214–18222
Kuznetsova E, Petrykin V, Sunde S, Krtil P (2015) Selectivity of nanocrystalline IrO2-based catalysts in parallel chlorine and oxygen evolution. Electrocatalysis 6:198–210
Yang KR, Matula AJ, Kwon G, Hong J, Sheehan SW, Thomsen JM, Brudvig GW, Crabtree RH, Tiede DM, Chen LX, Batista VS (2016) Solution structures of highly active molecular Ir water-oxidation catalysts from density functional theory combined with high-energy X-ray scattering and EXAFS spectroscopy. J Am Chem Soc 138:5511–5514
Fu HQ, Zhang L, Wang CW, Zheng LR, Liu PF, Yang HG (2018) 1D/1D hierarchical nickel sulfide/phosphide nanostructures for electrocatalytic water oxidation. ACS Energy Lett 3:2021–2029
Gu C, Hu S, Zheng X, Gao M-R, Zheng Y-R, Shi L, Gao Q, Zheng X, Chu W, Yao H-B, Zhu J, Yu S-H (2018) Synthesis of sub-2 nm iron-doped NiSe2 nanowires and their surface-confined oxidation for oxygen evolution catalysis. Angew Chem Int Ed 57:4020–4024
García-Mota M, Bajdich M, Viswanathan V, Vojvodic A, Bell AT, Nørskov JK (2012) Importance of correlation in determining electrocatalytic oxygen evolution activity on cobalt oxides. J Phys Chem C 116:21077–21082
Ye S, Wang J, Hu J, Chen Z, Zheng L, Fu Y, Lei Y, Ren X, He C, Zhang Q, Liu J (2021) Electrochemical construction of low-crystalline CoOOH nanosheets with short-range ordered grains to improve oxygen evolution activity. ACS Catal 11:6104–6112
Kong Q, Feng W, Xie X, Zhang S, Yuan X, Sun C (2018) Morphology-controlled synthesis of Co3O4 materials and its electrochemical catalytic properties towards oxygen evolution reaction. Catal Lett 148:3771–3778
Xu Y, Zhang F, Sheng T, Ye T, Yi D, Yang Y, Liu S, Wang X, Yao J (2019) Clarifying the controversial catalytic active sites of Co3O4 for the oxygen evolution reaction. J Mater Chem A 7:23191–23198
Chen Z, Kronawitter CX, Yeh Y-W, Yang X, Zhao P, Yao N, Koel BE (2017) Activity of pure and transition metal-modified CoOOH for the oxygen evolution reaction in an alkaline medium. J Mater Chem A 5:842–850
Zhang Z, Feng C, Wang D, Zhou S, Wang R, Hu S, Li H, Zuo M, Kong Y, Bao J, Zeng J (2022) Selectively anchoring single atoms on specific sites of supports for improved oxygen evolution. Nat Commun 13:2473
Gawande MB, Ariga K, Yamauchi Y (2021) Single-atom catalysts. Small 17:2101584
Kumar P, Kannimuthu K, Zeraati AS, Roy S, Wang X, Wang X, Samanta S, Miller KA, Molina M, Trivedi D, Abed J, Campos Mata MA, Al-Mahayni H, Baltrusaitis J, Shimizu G, Wu YA, Seifitokaldani A, Sargent EH, Ajayan PM, Hu J, Kibria MG (2023) High-density cobalt single-atom catalysts for enhanced oxygen evolution reaction. J Am Chem Soc 145:8052–8063
Mukherjee B (2021) First principles investigation on cobalt–tetracyanoquinodimethane monolayer for efficient Bi-functional single atom electrocatalyst. J Electroanal Chem 897:115602
Mukherjee B (2021) Highly efficient electrocatalyst for oxygen evolution reaction: DFT investigation on transition metal-tetracyanoquinodimethane monolayer. ChemistrySelect 6:609–616
Cai C, Wang M, Han S, Wang Q, Zhang Q, Zhu Y, Yang X, Wu D, Zu X, Sterbinsky GE, Feng Z, Gu M (2021) Ultrahigh oxygen evolution reaction activity achieved using Ir single atoms on amorphous CoOx nanosheets. ACS Catal 11:123–130
Dai Y, Yu J, Wang J, Shao Z, Guan D, Huang Y-C, Ni M (2022) Bridging the charge accumulation and high reaction order for high-rate oxygen evolution and long stable Zn-air batteries. Adv Funct Mater 32:2111989
Lei Z, Cai W, Rao Y, Wang K, Jiang Y, Liu Y, Jin X, Li J, Lv Z, Jiao S, Zhang W, Yan P, Zhang S, Cao R (2022) Coordination modulation of iridium single-atom catalyst maximizing water oxidation activity. Nat Commun 13:24
Zhang Z, Tan G, Kumar A, Liu H, Yang X, Gao W, Bai L, Chang H, Kuang Y, Li Y, Sun X (2023) First-principles study of oxygen evolution on Co3O4 with short-range ordered Ir doping. Mol Catal 535:112852
Shan J, Ye C, Jiang Y, Jaroniec M, Zheng Y, Qiao S-Z (2022) Metal-metal interactions in correlated single-atom catalysts. Sci Adv 8:eabo0762
Zheng Y, Gao R, Zheng L, Sun L, Hu Z, Liu X (2019) Ultrathin Co3O4 nanosheets with edge-enriched 111 planes as efficient catalysts for lithium-oxygen batteries. ACS Catal 9:3773–3782
Yan G, Wähler T, Schuster R, Schwarz M, Hohner C, Werner K, Libuda J, Sautet P (2019) Water on oxide surfaces: a triaqua surface coordination complex on Co3O4(111). J Am Chem Soc 141:5623–5627
Schwarz M, Faisal F, Mohr S, Hohner C, Werner K, Xu T, Skála T, Tsud N, Prince KC, Matolín V, Lykhach Y, Libuda J (2018) Structure-dependent dissociation of water on cobalt oxide. J Phys Chem Lett 9:2763–2769
Fickenscher G, Hohner C, Xu T, Libuda J (2021) Adsorption of D2O and CO on Co3O4(111): water stabilizes coadsorbed CO. J Phys Chem C 125:26785–26792
Kresse G, Furthmüller J (1996) Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput Mater Sci 6:15–50
Kresse G, Furthmüller J (1996) Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys Rev B 54:11169–11186
Blöchl PE (1994) Projector augmented-wave method. Phys Rev B 50:17953
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59:1758
Chadi DJ (1977) Spin-orbit splitting in crystalline and compositionally disordered semiconductors. Phys Rev B 16:790–796
Rossmeisl J, Logadottir A, Nørskov JK (2005) Electrolysis of water on (oxidized) metal surfaces. Chem Phys 319:178–184
Saal JE, Wang Y, Shang S, Liu Z-K (2010) Thermodynamic properties of Co3O4 and Sr6Co5O15 from first-principles. Inorg Chem 49:10291–10298
Wang L, Maxisch T, Ceder G (2007) A first-principles approach to studying the thermal stability of oxide cathode materials. Chem Mater 19:543–552
Henkelman G, Arnaldsson A, Jónsson H (2006) A fast and robust algorithm for Bader decomposition of charge density. Comput Mater Sci 36:354–360
Cai Z, Zhou D, Wang M, Bak S-M, Wu Y, Wu Z, Tian Y, Xiong X, Li Y, Liu W, Siahrostami S, Kuang Y, Yang X-Q, Duan H, Feng Z, Wang H, Sun X (2018) Introducing Fe2+ into nickel-iron layered double hydroxide: local structure modulated water oxidation activity. Angew Chem Int Ed 57:9392–9396
Nørskov JK, Rossmeisl J, Logadottir A, Lindqvist L, Kitchin JR, Bligaard T, Jónsson H (2004) Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J Phys Chem B 108:17886–17892
Tkalych AJ, Zhuang HL, Carter EA (2017) A density functional + U assessment of oxygen evolution reaction mechanisms on β-NiOOH. ACS Catal 7:5329–5339
Wu R, Liu D, Geng J, Bai H, Li F, Zhou P, Pan H (2022) Electrochemical reduction of CO2 on single-atom catalysts anchored on N-terminated TiN (111): low overpotential and high selectivity. Appl Surf Sci 602:154239
Wei G, Shen Y, Zhao X, Wang Y, Zhang W, An C (2022) Hexagonal phase Ni3Fe nanosheets toward high-performance water splitting by a room-temperature methane plasma method. Adv Funct Mater 32:2109709
Zhang Y, Zhang J, Huang J (2019) Potential-dependent volcano plot for oxygen reduction: mathematical origin and implications for catalyst design. J Phys Chem Lett 10:7037–7043
Rossmeisl J, Qu ZW, Zhu H, Kroes GJ, Nørskov JK (2007) Electrolysis of water on oxide surfaces. J Electroanal Chem 607:83–89
Hu S, Li W-X (2021) Sabatier principle of metal-support interaction for design of ultrastable metal nanocatalysts. Science 374:1360–1365
Xu J, Yu B, Zhao H, Cao S, Song L, Xing K, Zhou R, Lu X (2020) Oxygen-doped VS4 microspheres with abundant sulfur vacancies as a superior electrocatalyst for the hydrogen evolution reaction. ACS Sustain Chem Eng 8:15055–15064
Liu M, Li N, Cao S, Wang X, Lu X, Kong L, Xu Y, Bu X-H (2022) A “pre-constrained metal twins” strategy to prepare efficient dual-metal-atom catalysts for cooperative oxygen electrocatalysis. Adv Mater 34:2107421
Chen X, Shen S, Guo L, Mao SS (2010) Semiconductor-based photocatalytic hydrogen generation. Chem Rev 110:6503–6570
Tajuddin AAH, Wakisaka M, Ohto T, Yu Y, Fukushima H, Tanimoto H, Li X, Misu Y, Jeong S, Fujita J-I, Tada H, Fujita T, Takeguchi M, Takano K, Matsuoka K, Sato Y, Ito Y (2023) Corrosion-resistant and high-entropic non-noble-metal electrodes for oxygen evolution in acidic media. Adv Mater 35:2207466
Orville-Thomas WJ (1996) Atoms in molecules—a quantum theory: Richard F.W. Bader, Clarendon Press, Oxford, U.K. 1994, 438 pp., £25. J Mol Struct: THEOCHEM 360:175
Xu S-M, Liang X, Liu X, Bai W-L, Liu Y-S, Cai Z-P, Zhang Q, Zhao C, Wang K-X, Chen J-S (2020) Surface engineering donor and acceptor sites with enhanced charge transport for low-overpotential lithium–oxygen batteries. Energy Storage Mater 25:52–61
Jiao X, Hu Z, Zheng K, Zhu J, Wu Y, Zhang X, Hu J, Yan W, Zhu J, Sun Y, Xie Y (2022) Direct polyethylene photoreforming into exclusive liquid fuel over charge-asymmetrical dual sites under mild conditions. Nano Lett 22:10066–10072
Chen F, Ding J, Guo K, Yang L, Zhang Z, Yang Q, Yang Y, Bao Z, He Y, Ren Q (2021) CoNi alloy nanoparticles embedded in metal-organic framework-derived carbon for the highly efficient separation of xenon and krypton via a charge-transfer effect. Angew Chem Int Ed 60:2431–2438
Sharma HN, Sharma V, Hamzehlouyan T, Epling W, Mhadeshwar AB, Ramprasad R (2014) SOx oxidation kinetics on Pt(111) and Pd(111): first-principles computations meet microkinetic modeling. J Phys Chem C 118:6934–6940
Zhang B, Wang L, Cao Z, Kozlov SM, García de Arquer FP, Dinh CT, Li J, Wang Z, Zheng X, Zhang L, Wen Y, Voznyy O, Comin R, De Luna P, Regier T, Bi W, Alp EE, Pao C-W, Zheng L, Hu Y, Ji Y, Li Y, Zhang Y, Cavallo L, Peng H, Sargent EH (2020) High-valence metals improve oxygen evolution reaction performance by modulating 3d metal oxidation cycle energetics. Nat Catal 3:985–992
Acknowledgements
This work was supported by the National Key Research and Development Project (No.2022YFA1504001), and the Beijing Natural Science Foundation (No. Z210016). The authors thank the National Supercomputing Center in Shenzhen and the High-Performance Computing (HPC) Platform at Beijing University of Chemical Technology (BUCT) for providing part of the computational sources.
Author information
Authors and Affiliations
Contributions
WX, ZZ, HS, and YZ participated in the preliminary background investigation. WX carried out DFT calculations, processed the results, and wrote the original draft. YK provided writing review and editing. YL provided resources, writing review and editing. And all authors have given approval to the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing financial interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, W., Zhang, Z., Sun, H. et al. First-Principles Study of Oxygen Evolution Reaction on Ir with Different Coordination Numbers Anchoring at Specific Sites of Co3O4 (111) Surface. Catal Lett 154, 2488–2502 (2024). https://doi.org/10.1007/s10562-023-04486-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-023-04486-w