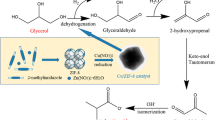
Abstract
Catalytic conversion of glycerol to lactic acid has been considered as one of the effective ways to solve the problem of glycerol surplus. In this paper, we reported a facile synthesis of Cu/ZnO/C by direct calcination of Cu-supported zeolitic imidazolate framework(ZIF-8), and the performance of the catalysts for converting glycerol to lactic acid were investigated. The properties of Cu/ZnO/C catalysts were characterized by XRD, BET, SEM and TG techniques. The results showed that nano-copper was distributed uniformly on the porous carbon carrier. The zinc oxide after thermal treatment provided abundant metal active sites and had synergistic effect with nano copper. In addition, the lactic acid selectivity of 84% was obtained at the glycerol conversion of 95.1% at 230 °C, 1.5 MPa and 15% copper loading for 6 h reaction. Moreover, the yield of lactic acid still remained above 80% after being recycled four times.
Graphical Abstract















Similar content being viewed by others
References
Ennetta R, Soyhan HS, Koyunoglu C et al (2022) Current technologies and future trends for biodiesel production: a Review. Arab J Sci Eng 47:15133–15151
Varanda MG, Pinto G, Martins F (2011) Life cycle analysis of biodiesel production. Fuel Process Technol 92:1087–1094
Moklis MH, Cheng S, Cross JS (2023) Current and future trends for crude glycerol upgrading to high value-added products. Sustainbility 15(4):2979
Quispe CAG, Coronado CJR, Carvalho JA (2013) Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew Sustain Energy Rev 27:475–493
Long YD, Fang Z (2013) Hydrothermal conversion of glycerol to chemicals and hydrogen: review and perspective. Biofuels, Bioprod Biorefin 6(6):686–702
Jiang H, Ma JS, Zeng BQ et al (2022) Research progress in 1, 3-propanediol production by fermenting crude glycerol. Biotechnol Bull 38(10):45–53
García-Sancho C, Cecilia JA, Moreno-Ruiz A et al (2015) Influence of the niobium supported species on the catalytic dehydration of glycerol to acrolein. Appl Catal B 179:139–149
Wang D, Zhang X, Cong X et al (2018) Influence of Zr on the performance of Mg-Al catalysts via hydrotalcite-like precursors for the synthesis of glycerol carbonate from urea and glycerol. Appl Catal A 555:36–46
Wang J, Yao G, Jin F (2007) One-pot catalytic conversion of carbohydrates into alkyl lactates with Lewis acids in alcohols. Mol Catal 435:82–90
Arcanjo MRA, Silva IJ, Cavalcante CL et al (2009) Glycerol valorization: conversion to lactic acid by heterogeneous catalysis and separation by ion exchange chromatography. Biofuels, Bioprod Biorefin 14:357–370
Lasprilla AJR, Martinez GAR, Lunelli BH et al (2012) Polylactic acid synthesis for application in biomedical devices: a review. Biotechnol Adv 30(1):321–328
Kim J, Kim YM, Lebaka VR (2022) Lactic acid for green chemical industry: recent advances in and future prospects for production technology, recovery, and applications. Fermentation-Basel 8(11):609
Djukic-Vukovic AP, Mojovic LV, Vukašinović-Sekulić MS (2012) Effect of different fermentation parameters on L-lactic acid production from liquid distillery stillage. Food Chem 134:10381043
Li XS, Gao DC, Wang LM et al (2017) Research progress on preparation of D-lactic acid by fermentation. Contemp Chem Ind 46(8):1659–1662
Li ZJ, Ren D, Su WT et al (2011) Breeding of high yield L-lactic acid strain and optimization of fermentation conditions. J Sichuan Univ 48(2):451–456
Ghaffar T, Irshao M, Anwar Z et al (2014) Recent trends in lactic acid biotechnology: a brief review on production to purification. J Radiat Res Appl Sci 7(2):222–229
Oh H, Wee YJ, Yun JS et al (2005) Lactic acid production from agricultural resources as cheap raw materials. Biores Technol 196:1492–1498
Murillo B, Zornoza B, De La Iglesia O et al (2016) Chemocatalysis of sugars to produce lactic acid derivatives on zeolitic imidazolate frameworks. J Catal 334:60–67
Auneau F, Aranil S, Besson M et al (2012) Heterogeneous transformation of glycerol to lactic acid. Top Catal 55(7/10):474–479
Kishida H, Jin F, Zhou Z et al (2005) Conversion of glycerin into lactic acid by alkaline hydrothermal reaction. Chem Lett 34:1560–1561
Dutta M, Das K, Prathapa SJ et al (2020) Selective and high yield transformation of glycerol to lactic acid using NNN pincer ruthenium catalysts. Chem Commun 56:9886–9889
Evans CD, Douthwaite M, Carter JH et al (2020) Enhancing the understanding of the glycerol to lactic acid reaction mechanism over AuPt/TiO2 under alkaline conditions. J Chem Phys 152:134705
Shen L, Yu Z, Zhang D et al (2019) Glycerol valorization to lactic acid catalyzed byhydroxyapatite-supported palladium particles. J Chem Technol Biotechnol 94:204–215
Bruno AM, Chagas CA, Souza MMVM et al (2018) Lactic acid production from glycerol in alkaline medium using Pt-based catalysts in continuous flow reactionsystem. Renew Energy 118:160–171
Wang C, Zhang X, Li J et al (2021) Gold nanoparticles on nanosheets derived from layered rare-earth hydroxides for catalytic glycerol-to-lactic acid conversion. ACS Appl Mater Interfaces 13:522–530
Shen Y, Zhang S, Li H et al (2010) Efficient synthesis of lactic acid by aerobic oxidation of glycerol on Au-Pt/TiO2 catalysts. Chem-A Euro J 16:7368–7371
Shen LQ, Zhou X, Zhang CX et al (2019) Functional characterization of bimetallic CuPdx nanoparticles in hydrothermal conversion of glycerol to lactic acid. Food Chem 43(8):12931
Qiu L, Yin HX, Yin HB et al (2018) Catalytic conversion of glycerol to lactic acid over hydroxyapatite-supported metallic Ni-0 nanoparticles. J Nanosci Nanotechnol 18:4734–4745
Yin HX, Yin HB, Wang AL et al (2018) Catalytic conversion of glycerol to lactic acid over graphite-supported nickel nanoparticles and reaction kinetics. J Ind Eng Chem 57:226–235
Xiu Z, Wang H, Cai C et al (2020) Ultrafast glycerol conversion to lactic acid over magnetically recoverable Ni−NiOx@C catalysts. Ind Eng Chem Res 59:9912–9925
Torres S, Palacio R, López D (2021) Support effect in Co3O4-based catalysts for selective partial oxidation of glycerol to lactic acid. Appl Catal A 621:118199
Qiu K, Shu YX, Zhang J et al (2022) Effective and stable zeolite imidazole framework-supported copper nanoparticles (Cu/ZIF-8) for glycerol to lactic acid. Catal Lett 152:172–186
Zhang JJ, Wu XY, Chen JZ et al (2023) Efficient and stable Cu-Cu2O@NC catalysts for selective catalytic conversion of glycerol to lactic acid. ChemCatChem. https://doi.org/10.1002/cctc.202201139
Wang A, Xu Q, Yin H (2022) Synthesis of lactic acid starting from glycerol catalyzed by CaO-supported CuO and metallic Cu catalysts in Ca(OH)2 aqueous solution. React Kinet Mech Catal 135:3205–3221
Xia W, Mahmood A, Zou R et al (2015) Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ Sci 8(7):1837–1866
Zhang S, Liu H, Liu P et al (2015) A template-free method for stable CuO hollow microspheres fabricated from a metal organic framework (HKUST-1). Nanoscale 7(21):9411–9415
Tahmouresilerd B, Larson PJ, Unruh DK et al (2018) Make room for iodine: systematic pore tuning of multivariate metal-organic frameworks for the catalytic oxidation of hydroquinones using hypervalent iodine. Catal Sci Technol 8(17):4349–4357
Liu J, Chen L, Cui H et al (2014) Cheminform abstract: Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. J Cheminfomatics 45(43):6011–6061
Xie MH, Wang Y, Li RF et al (2018) A multifunctional co-based metal-organic framework: heterogeneous catalysis, chemiluminescence sensing and moisture-dependent solvatochromism. Dalton Trans 47(35):12406–12413
Li ZF, Shen Y, Zhang Q et al (2022) Budget MOF-derived catalyst to realize full conversion from furfural to furfuryl alcohol. Mol Catal 518:112092
Xue Y, Li CJ, Zhou XX et al (2022) MOF-derived Cu/Bi Bi-metallic catalyst to enhance selectivity toward formate for CO2 electroreduction. ChemElectroChem. https://doi.org/10.1002/celc.202101648
Zhong W, Liu H, Bai C et al (2015) Base-free oxidation of alcohols to esters at room temperature and atmospheric conditions using nanoscale Co-based catalysts. ACS Catal 5:1850–1856
Jiang M, Cao XP, Zhu DD et al (2016) Hierarchically porous n-doped carbon derived from ZIF-8 nanocomposites for electrochemical applications. Electrochim Acta 196:699–707
Cao PK, Liu YM, Quan X et al (2019) Nitrogen-doped hierarchically porous carbon nanopolyhedras derived from core-shell ZIF-8@ZIF-8 single crystals for enhanced oxygen reduction reaction. Catal Today 327:366–373
Park SK, Park JS, Kang YC (2018) Metal-organic-framework-derived n-doped hierarchically porous carbon polyhedrons anchored on crumpled graphene balls as efficient selenium hosts for high-performance lithium-selenium batteries. ACS Appl Mater Interfaces 10(19):16531–16540
Zhang JP, Liu XY, Chen WK et al (2020) N configuration control of N-doped carbon for stabilizing Cu nanoparticles: the synergistic effects on oxy-carbonylation of methanol. Carbon 158:836–845
Dahal B, Chae SH, Muthurasu A et al (2020) An innovative synthetic approach for core-shell multiscale hierarchically porous boron and nitrogen codoped carbon nanofibers for the oxygen reduction reaction. J Power Sour 453:11
Chen B, Ma G, Zhu Y et al (2016) Metal-organic-framework-derived bi-metallic sulfide on N, S-codoped porous carbon nanocomposites as multifunctional electrocatalysts. J Power Sources 334:112–119
Choi M, Cho HS, Srivastava R et al (2006) Amphiphilic organosilane-directed synthesis of crystalline zeolite with tunable mesoporosity. Nat Mater 5(9):718–723
Fox DM, Harris RH, Bellayer S et al (2011) The pillaring effect of the 1, 2-dimethyl-3(benzyl ethyl iso-butyl poss) imidazolium cation in polymer/montmorillonite nanocomposites. Polymer 52(23):5335–5343
Hao Y, Zhang S, Tao P et al (2020) Pillaring effect of k ion anchoring for stable V2O5-based zinc-ion battery cathodes. Chemnanomat 6(5):797–805
Acknowledgements
This work was financially supported by National Key R&D Program of China (No.2019YFB1504003).
Funding
National Key Research and Development Program of China, Grant No. 2019YFB1504003
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest or personal relationships that may affect the work reported in this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Qiu, K., Sun, D. et al. Cu/ZIF-8 Derived Cu/ZnO/C Catalysts for Efficient Conversion of Glycerol to Lactic Acid. Catal Lett 154, 1309–1321 (2024). https://doi.org/10.1007/s10562-023-04393-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-023-04393-0