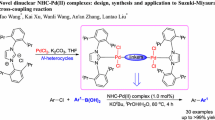
Abstract
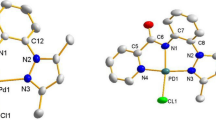
Three new pincer type palladium(II) complexes 1–3 synthesized by reacting palladium precursor [PdCl2(PPh3)2] with ONO heterocyclic hydrazone ligands H2L1-3 in equimolar quantities were well characterized using single-crystal XRD, analytical and spectroscopic data. These complexes were examined as homogeneous catalysts for the Suzuki–Miyaura cross-coupling of 3-bromochromone with a range of arylboronic acids in ethanol medium. Catalytic transformations progressed smoothly under aerobic conditions with 0.01 mol% of catalyst load at room temperature to afford an array of 3-arylated chromones (6a–o). This catalyst was reused up to five cycles and favourably this protocol does not require any external oxidant, additives and phase transfer reagents.
Graphical Abstract










Similar content being viewed by others
References
Silva CFM, Batista VF, Pinto DCGA, Silva AMS (2018) Expert Opin Drug Dis 13:795–798
Veitch NC (2007) Nat Prod Rep 24:417–464
Boland GM, Donnelly DMX (1998) Nat Prod Rep 15:241–260
Ha H, Lee YS, Lee JH (2006) Arch Pharm Res 29:96–101
Van Erp-Baart M-AJ, Brants HAM, Kiely M (2003) Br J Nutr 89:S25–S30
Ding K, Wang S (2005) Tetrahedron Lett 46:3707–3709
Zhang YC, Albrecht D, Bomser J (2003) J Agr Food Chem 51:7611–7616
Hendrich S (2002) J Chromatogr B 777:203–210
Awoniyi CA, Roberts D, Rao Veeramachaneni DN (1998) Fertil Steril 70:440–447
Křížová L, Dadáková K, Kašparovská J, Kašparovský T (2019) Molecules 24:1076
Beggs CJ, Stolzer-Jehle A, Wellmann E (1985) Plant Physiol 79:630–634
Samanta Amalesh, Das Gouranga DSK (2011) Int J Pharm Sci Tech 6:12–35
Miadoková E (2009) Interdiscip Toxicol 2:211–218
Morel C, Stermitz FR, Tegos G, Lewis K (2003) J Agr Food Chem 51:5677–5679
Alesawy MS, Abdallah AE, Taghour MS (2021) Molecules 26:2806
Lee JH, Renita M, Fioritto RJ (2004) J Agric Food Chem 52:2647–2651
Barve V, Ahmed F, Adsule S (2006) J Med Chem 49:3800–3808
Yu J, Bi X, Yu B, Chen D (2016) Nutrients 8:1–16
Toukam PD, Tagatsing MF, Tchokouaha Yamthe LR et al (2018) Phytochem Lett 28:69–75
Shah SK, Jhade DN (2016) Int J Green Pharm 10:S204–S210
Soni P, Choudhary R, Bodakhe SH (2019) J Integr Med 17:374–382
Zhao J, Cheng YY, Fan W et al (2015) CNS Neurosci Ther 21:61–70
Wu Q, Wang M, Sciarappa WJ, Simon JE (2004) J Agr Food Chem 52:2763–2769
Chen J, Wu Y, Yang C et al (2017) Food and Funct 8:4414–4420
Hikal WM, Baeshen RS, Said-Al Ahl HAH (2017) Cogent Biol 3:1404274
Cao H, Ou J, Chen L et al (2019) Crit Rev Food Sci Nutr 59:3371–3379
Li W, Liu F, Zhang P (2008). J Chem Res. https://doi.org/10.3184/030823408X382135
Chien JT, Hsieh HC, Kao TH, Chen BH (2005) Food Chem 91:425–434
Marais JPJ, Ferreira D, Slade D (2005) Phytochem 66:2145–2176
Paquette LA, Stucki H (1966) J Org Chem 31:1232–1235
Tsoi YT, Zhou Z, Chan ASC, Yu WY (2010) Org Lett 12:4506–4509
Boström J, Brown DG, Young RJ, Keserü GM (2018) Nat Rev Drug Discov 17:709–727
Polshettiwar V, Decottignies A, Len C, Fihri A (2010) Chemsuschem 3:502–522
Astruc D (2011) Anal Bioanal Chem 399:1811–1814
Van Koten G (2013) Top Organomet Chem 40:1–20
Yukio hoshino N miyaura and A suzuki (1988) Bull Chem Sco Jpn. 3008–3010
Wan JP, Tu Z, Wang Y (2019) Chem Eur J 25:6907–6910
Silva VLM, Soengas RG, Silva AMS (2020) Molecules 25(7):1564
Chang YT, Liu LJ, Peng WS (2021) J Chin Chem Soc 68:469–475
Firinci E, Firinci R, Sevincek R (2022) J Organomet Chem 978:122471
Layek S, Agrahari B, Ganguly R (2020) Applied Organomet Chem 34:1–14
Vignesh A, Shalini C, Dharmaraj N (2019) Eur J Inorg Chem 2019:3869–3882
Vogel AI (1989) Textbook of practical organic chemistry, 5th edn. Longman, London
Sathyadevi P, Krishnamoorthy P, Butorac RR, Cowley AH, Bhuvanesh NSP, Dharmarai N, Dalton Trans (2011) 9690.
APEX3 Program for data collection on area detectors. BRUKER AXS Inc., Madison
SADABS, Sheldrick GM. Program for Absorption Correction of Area Detector Frames. BRUKER AXS Inc., Madison
Sheldrick GM (2008) Acta Cryst A 64:112–122. Sheldrick GM (2015) Acta Cryst A 71:3–8. Sheldrick GM (2015) Acta Cryst C 71:3–8. XT, XS, BRUKER AXS Inc., Madison
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Cryst 42:339–341
Spek AL (2003) J Appl Cryst 36:7–13; Spek AL (2008) Utrecht University, Utrecht
Felpin FX, Lory C, Sow H, Acherar S (2007) Tetrahedron 63:3010–3016
Zhang Z, Qiao J, Wang D (2014) Mol Divers 18:245–251
Babu GN, Pal S (2016) J Organomet Chem 9:805
Rao ARB, Pal S (2012) J Organomet Chem 67:701
Rao ARB, Pal S (2013) J Organomet Chem 67:731
Anitha P, Manikandan R, Viswanathamurthi P (2015) J Coord Chem 68:3537
Tamizh MM, Karvembu R (2012) Inorg Chem Commun 25:30
Ayyannan G, Veerasamy P, Mohanraj M, Raja G, Manimaran A, Velusamy M, Bhuvanesh N, Nandhakumar R, Jayabalakrishnan C (2017) Appl Organomet Chem 31:1
Albert J, Gonzalez A, Granell J, Moragas R, Puerta C, Valerga P (1997) Organometallics 16:3775
Das S, Pal S (2006) J Organomet Chem 691:2575–2583
Nava DR, Hernández AÁ, Rheingold AL, Suarez-Castillo OR, Mendoza- Espinosa D (2019) Dalton Trans 48:3214–3222
Martin AR, Yang Y (1993) Acta Chem Scand 47:221–230
Acknowledgements
Mrs. C. Shalini thanks the Department of Science and Technology (DST), New Delhi, India, for the award of Promotion of University Research and Scientific Excellence (PURSE) – Phase – II fellowship (Official memorandum Number: BU/DST PURSE (II)/Appointment/13).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shalini, C., Dharmaraj, N., Bhuvanesh, N.S.P. et al. Palladium(II) Pincer Type Complexes Containing ONO Donor Heterocyclic Hydrazones: Synthesis, Structure and Catalytic Activity Towards the Suzuki–Miyaura Cross-Coupling of 3-Bromochromone and Arylboronic Acids via C–Br Activation. Catal Lett 154, 132–143 (2024). https://doi.org/10.1007/s10562-023-04276-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-023-04276-4