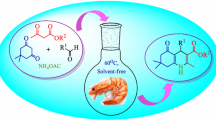
Abstract
A highly efficient recoverable and reusable heterogeneous Zn-catalyst, chitosan supported ZnSO4. 7H2O (designated as Zn@CS) is synthesized via a simple synthetic route. Low catalyst loading, need of aqueous reaction media and sharp reduction of time requirement in comparison to the previously reported methodologies are some special features of the established Zn catalyzed direct azide-alkyne cycloaddition reaction. Zn@CS-catalyst is also suitable for multicomponent synthesis of 4-aryl-NH-1,2,3-triazoles from different derivatives of benzaldehyde. An inexpensive and environmentally benign deep eutectic solvent (DES), ChCl:PEG-400:Glycerol as a suitable reaction media accelerates these reactions leading to excellent yields of NH-triazoles. Based on literature report a plausible mechanism has been suggested for ZnAAC reaction.
Graphical Abstract









Similar content being viewed by others
References
Balaban AT, Oniciu DC, Katritzky AR (2004) Aromaticity as a cornerstone of heterocyclic chemistry. Chem Rev 104:2777
Fan WQ (1996) Katritzky AR. Comprehensive heterocyclic chemistry II. Elsevier Science, Oxford, p 1
Alvarez R, Velazquez S, San-Felix A, Aquaro S, De Clercq E, Perno CF, Karlsson A, Balzarini J, Camarasa MJ (1994) 1,2,3-Triazole-[2’,5’-bis-O-(tert-butyldimethylsilyl)-beta-D- ribofuranosyl]-3’-spiro-5"-(4"-amino-1",2"-oxathiole 2",2"-dioxide) (TSAO) analogues: synthesis and anti-HIV-1 activity. J Med Chem 3:4185
Genin MJ, Allwine DA, Anderson DJ, Barbachyn MR, Emmert DE, Garmon SA, Graber DR, Grega KC, Hester JB, Hutchinson DK, Morris J, Reischer RJ, Ford CW, Zurenko GE, Hamel JC, Schaadt RD, Stapert D, Yagi BH (2000) Substituent effects on the antibacterial activity of nitrogen-carbon-linked (azolylphenyl)oxazolidinones with expanded activity against the fastidious gram-negative organisms Haemophilus influenzae and Moraxella catarrhalis. J Med Chem 43:953
Buckle DR, Rockell CJM (1982) Studies on v-triazoles. Part 4. The 4-methoxybenzyl group, a versatile N-protecting group for the synthesis of N-unsubstituted v-triazoles. J Chem Soc Perkin Trans 1:627
Brockunier LL, Parme ER, Ok HO, Candelore MR, Cascieri MA, Colwell LF, Deng L, Feeney WP, Forrest MJ, Hom GJ, MacIntyre DE, Tota L, Wyvratt MJ, Fisher MH, Weber AE (2000) Human beta3-adrenergic receptor agonists containing 1,2,3-triazole-substituted benzenesulfonamides. Bioorg Med Chem Lett 10:2111
Kolb HC, Finn MG, Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed 40:2004
Huisgen R (1963) 1,3-dipolar cycloadditions past and future. Angew Chem Int Ed 2:565
Huisgen R (1963) 1,3-dipolar cycloadditions past and future. Angew Chem Int Ed 2:633
Zhang X, Hsung RP, Li HA (2007) A triazole-templated ring-closing metathesis for constructing novel fused and bridged triazoles. Chem Commun 23:2420–2422
Gonda Z, Novak Z (2010) Highly active copper-catalysts for azide-alkynecycloaddition. Dalton Trans 39:726
Peddibhotla S, Dang Y, Liu JO, Romo D (2007) Simultaneous arming and structure/activity studies of natural products employing O-H insertions: an expedient and versatile strategy for natural products-based chemical genetics. J Am Chem Soc 129:1222
Beckmann HSG, Wittmann V (2007) One-pot procedure for diazo transfer and azide- alkyne cycloaddition: triazole linkages from amines. Org Lett 9:1
Barral K, Moorhouse AD, Moses JE (2007) Efficient conversion of aromatic amines into azides: a one-pot synthesis of triazole linkages. Org Lett 9:1809
Hagiwara H, Sasaki H, Hoshi T, Suzuki T (2009) Sustainable click reaction catalyzed by supported ionic liquid catalyst (Cu-SILC). Synlett 4:643
Chassaing S, Kumarraja M, Sani Souna Sido A, Pale P, Sommer J (2007) Click chemistry in CuI-Zeolites: the huisgen [3 + 2]-cycloaddition. Org Lett 9:883
Lipshutz BH, Taft BR (2006) Heterogeneous copper-in-charcoal-catalyzed click chemistry. Angew Chem 118:8415
Jlalia I, Elamari HF, Meganem Herscovici J, Girard C (2008) Copper(I)-doped Wyoming’s montmorillonite for the synthesis of disubstituted 1,2,3-triazoles. Tetrahedron Lett 49:6756
Urbani CN, Bell CA, Whittaker MR, Monteiro MJ (2008) Convergent synthesis of second generation AB-type miktoarm dendrimers using “Click” chemistry catalyzed by copper wire. Macromolecules 41:1057
Duran Pachon L, Van Maarseveen JH, Rothenberg G (2005) Click chemistry: copper clusters catalyse the cycloaddition of azides with terminal alkynes. Adv Synth Catal 347:811
Molteni G, Bianchi CL, Marinoni G, Santo N, Ponti A (2006) Cu/Cu-oxide nanoparticles as catalyst in the “click” azide–alkyne cycloaddition. New J Chem 30:1137
Rostovtsev VV, Green L, Fokin VV, Sharpless KB (2002) A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “Ligation” of azides and terminal alkynes. Angew Chem 41:2596
McNulty J, Keskar K, Vemula R (2011) The first well-defined silver(I)-complex-catalyzed cycloaddition of azides onto terminal alkynes at room temperature. Chem Eur J 17:14727
Salam N, Sinha A, Roy AS, Mondal P, Jana NR, Islam SM (2014) Synthesis of silver–graphene nanocomposite and its catalytic application for the one-pot threecomponent coupling reaction and one-pot synthesis of 1,4-disubstituted 1,2,3-triazoles in water. RSC Adv 4:10001
Ali AA, Chetia M, Saikia B, Saikia PJ, Sarma D (2015) AgN(CN)2/DIPEA/H2O-EG: a highly efficient catalytic system for synthesis of 1,4-disubstituted-1,2,3 triazoles at room temperature. Tetrahedron Lett 56:5892
Sultana J, Khupse ND, Chakrabarti S, Chattopadhyay P, Sarma D (2019) Ag2CO3-catalyzed cycloaddition of organic azides onto terminal alkynes: a green and sustainable protocol accelerated by aqueous micelles of CPyCl. TetrahedronLett 60:1117
Garg A, Khupse N, Bordoloi A, Sarma D (2019) Ag–NHC anchored on silica: an efficient ultra-low loading catalyst for regioselective 1,2,3-triazole synthesis. New J Chem 43:19331
Arado OD, Monig H, Wagner H, Franke JH, Langewisch G, Held PA, Studer A, Fuchs H (2013) On-surface azide alkyne cycloaddition on Au(111). ACS Nano 7:8509
Boominathan M, Pugazhenthiran N, Nagaraj M, Muthusubramanian S, Murugesan S, Bhuvanesh N (2013) Nanoporous titania-supported gold nanoparticle-catalyzed green synthesis of 1,2,3-triazoles in aqueous medium. ACS Sustain Chem Eng 1:1405
Rao HSP, Chakibanda G (2014) Raney Ni catalyzed azide-alkyne cycloaddition reaction. RSC Adv 4:46040
Meng X, Xu X, Gao T, Chen B (2010) Zn/C-catalyzed cycloaddition of azides and aryl alkynes. Eur J Org Chem 2010:5409–5414
Smith CD, Greaney MF (2013) Zinc mediated azidealkyne ligation to 1,5- and 1,4,5-substituted 1,2,3-triazoles. Org Lett 18:4826
Morozova MA, Yusubov MS, Kratochvil B, Eigner V, Bondarev AA, Yoshimura A, Saito A, Zhdankin VV, Trusova ME, Postnikov P (2017) Regioselective Zn(OAc)2-catalyzed azide–alkyne cycloaddition in water: the green click-chemistry. Org Chem Front 4:978
Sadeghi B, Hassanabadi A, Kamali M (2012) ZnO nanoparticles: efficient and versatile reagents for synthesis of 1,4-disubstituted 1,2,3-triazoles. J Chem Res 36:9
Zhu LL, Xu XQ, Shi JW, Chen BL, Chen Z (2016) N2-selective iodofunctionalization of olefins with NH-1,2,3-triazoles to provide N2-alkyl-substituted 1,2,3-triazoles. J Org Chem 81:3568
Jin T, Kamijo S, Yamamato Y (2004) Copper-catalyzed synthesis of N-unsubstituted 1,2,3-triazoles from nonactivated terminal alkynes. Eur J Org Chem 2004:3789
Barluenga J, Valdés C, Beltrán G, Escribano M, Aznar F (2006) Developments in Pd catalysis: synthesis of 1H–1,2,3-triazoles from sodium azide and alkenyl bromides. Angew Chem Int Ed 45:6893
Zhang H, Dong DZ, Wang ZL (2016) Direct synthesis of N-unsubstituted 4-aryl-1,2,3-triazoles mediated by amberlyst-15. Synthesis 48:131
Payra S, Saha A, Banerjee S (2018) On water Cu@g-C3N4 catalyzed synthesis of NH-1,2,3-triazoles via [2+3] cycloadditions of nitroolefins/alkynes and sodium azide. Chem Cat Chem 10:5468
Hu Q, Liu Y, Deng X, Li Y, Chen Y (2016) Aluminium(III) chloride-catalyzed three-component condensation of aromatic aldehydes, nitroalkanes and sodium azide for the synthesis of 4-Aryl-NH-1,2,3-triazoles. Adv Synth Catal 358:1689
Hui R, Zhao M, Chen M, Ren Z, Guan Z (2017) One-pot synthesis of 4-Aryl-NH-1,2,3-triazoles through three-component reaction of aldehydes, nitroalkanes and NaN3. Chin J Chem 35:1808
Daraie M, Heravi MM, Sarmasti N (2020) Synthesis of polymer-supported Zn(II) as a novel and green nanocatalyst for promoting click reactions and using design of experiment for optimization of reaction conditions. J Macromol Sci Part A: Pure Appl Chem 57:488
Qiu Y, Qin Y, Ma Z, Xia W (2014) Chitosan-supported zinc nitrate: preparation and catalyst for condensation reaction of aldehydes, amines, and terminal alkynes leading to the formation of propargylamines. Chem Lett 43:1284
Han XY, Ma YF, Lv MY, Wu ZP, Qian LC (2014) Chitosan-zinc chelate improves intestinal structure and mucosal function and decreases apoptosis in ileal mucosal epithelial cells in weaned pigs. British J Nutrition 111:1405
Phukan P, Agarwal S, Deori K, Sarma D (2020) Zinc oxide nanoparticles catalysed one pot three component reaction: a facile synthesis of 4 aryl NH 1,2,3 triazoles. Catal Lett 150:2208
Garg A, Sarma D, Ali AA (2020) Microwave assisted metal-free approach to access 1,2,3-triazoles through multicomponent synthesis. Curr Res Gr Sustain Chem 3:100013
Mekahlia S, Bouzid B (2009) Chitosan-Copper (II) complex as antibacterial agent: synthesis, characterization and coordinating bond- activity correlation study. Phys Procedia 2:1045
Varma AJ, Deshpande SV, Kennedy JF (2004) Metal complexation by chitosan and its derivatives: a review. Carbohyd Polym 55:77
Wang X, Du Y, Liu H (2004) Preparation, characterization and antimicrobial activity of chitosan–Zn complex. Carbohyd Polym 56:21
Kumar S, Nigam N, Gosh T, Duta PK, Yadav RS, Pandey AC (2010) Preparation, characterization and optical properties of a chitosan-anthraldehyde crosslinkable film. J Applied Polym Sci 115:3056
Adewuyi S, Bisiriyu IO, Akinremi CA (2015) Zinc (II) metal ion complexes of chitosan: towardheterogeneous-active catalysts for the polymerization of vinyl acetate. Ife J Sc 17:749
Durand E, Lecomte J, Villeneuve P (2013) Deep eutectic solvents: synthesis, application, and focus on lipase-catalyzed reactions. Eur J Lipid Sci Technol 115:379
Khandelwal S, Tailor YK, Kumar M (2016) Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J Mol Liq 215:345
Acknowledgements
The authors would like to acknowledge DST, New Delhi, India for a research grant [No. EMR/2016/002345]. The financial assistance of the DST-FIST and UGC-SAP program to the Department of Chemistry, Dibrugarh University is also gratefully acknowledged. We are also thankful to Dibrugarh University for providing Dibrugarh University Research Fellowship (DURF). The authors acknowledge CSIC, Dibrugarh University for NMR measurements and Dibrugarh University for providing all infrastructural facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflict to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sultana, J., Garg, A., Kulshrestha, A. et al. Zn@CS: An Efficient Cu-Free Catalyst System for Direct Azide-Alkyne Cycloadditions and Multicomponent Synthesis of 4-Aryl-NH-1,2,3-triazoles in H2O and DES. Catal Lett 153, 3516–3526 (2023). https://doi.org/10.1007/s10562-022-04248-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04248-0