Abstract
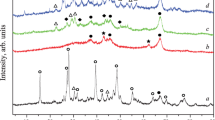
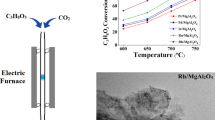
Lanthanum hexaaluminate (LHA) supported NiO catalysts (xNi/LHA) with varied mass loading of Ni (x = 15, 20, 25, 30 wt%) were prepared by impregnating NiO nanoparticles (NPs) on LHA support that synthesized through urea combustion method. The catalysts were characterized by high-resolution transmission electron microscope (HRTEM), temperature programmed reduction (TPR), temperature programmed desorption (TPD), etc. The results showed that NiO NPs were dispersed densely on the LHA surface. The 25Ni/LHA catalyst provided the optimal ammonia conversion of 85.75% with a space velocity of 30,000 ml gcat−1 h−1 at 600 °C, corresponding to the hydrogen production rate of 28.73 mmol H2 gcat−1 min−1. The strong alkalinity of LHA could modify the interaction between the active sites and the nitrogen of ammonia, which was conducive to the ammonia decomposition reaction. The high performance of the catalyst represented a feasible approach towards the application of ammonia as hydrogen carrier to produce hydrogen.
Graphical Abstract









Similar content being viewed by others
References
Zeng R, Feller M, Ben-David Y et al (2017) Hydrogenation and hydrosil-ylation of nitrous oxide homogeneously catalyzed by a metal complex. J Am Chem Soc 139:5720–5723. https://doi.org/10.1021/jacs.7b02124
Chen L, Song Y, Liu Y et al (2020) NiCoP nanoleaves array for electrocatalytic alkaline H2 evolution and overall water splitting. J Energy Chem 50:395–401. https://doi.org/10.1016/j.jechem.2020.03.046
Li Q, Wang Y, Zeng J et al (2021) Phosphating-induced charge transfer on CoO/CoP interface for alkaline H2 evolution. Chin Chem Lett 32:3355–3258. https://doi.org/10.1016/j.cclet.2021.03.063
Liu Y, Feng Q, Liu W et al (2018) Boosting interfacial charge transfer for alkaline hydrogen evolution via rational interior Se modification. Nano Energy 81:105641. https://doi.org/10.1016/j.nanoen.2020.105641
Song C, Liu Y, Wang Y et al (2021) Highly efficient oxygen evolution and stable water splitting by coupling NiFe LDH with metal phosphides. Sci China Mater 64:1662–1670. https://doi.org/10.1007/s40843-020-1566-6
Wang M, Wang Z, Gong X et al (2014) The intensification technologies to water electrolysis for hydrogen production - A review. Renew Sustain Energy Rev 29:573–588. https://doi.org/10.1016/j.rser.2013.08.090
Schüth F, Palkovits R, Schlögl R et al (2012) Ammonia as a possible element in an energy infrastructure: catalysts for ammonia decomposition. Energy Environ Sci 5:6278–6289. https://doi.org/10.1039/C2EE02865D
Lucentini I, Garcia X, Vendrell X et al (2021) Review of the decomposition of ammonia to generate hydrogen. Ind Eng Chem Res 60:18560–18611. https://doi.org/10.1021/acs.iecr.1c00843
Huang C, Yu Y, Yang J et al (2019) Ru/La2O3 catalyst for ammonia decomposition to hydrogen. Appl Surf Sci 476:928–936. https://doi.org/10.1016/j.apsusc.2019.01.112
Lucentini I, Casanovas A, Llorca J (2019) Catalytic ammonia decomposition for hydrogen production on Ni, Ru and Ni Ru supported on CeO2. Int J Hydrogen Energy 44:12693–12707. https://doi.org/10.1016/j.ijhydene.2019.01.154
Zhao J, Xu S, Wu H et al (2019) Metal-support interactionson Ru/CaAlOx catalysts derived from structural reconstruction of Ca-Al layered double hydroxides for ammonia decomposition. Chem Commun 55:14410–14413. https://doi.org/10.1039/C9CC05706D
Li L, Chen F, Shao J et al (2016) Attapulgite clay supported Ni nanoparticles encapsulated by porous silica: thermally stable catalysts for ammonia decomposition to COx free hydrogen. Int J Hydrogen Energy 41:21157–21165. https://doi.org/10.1016/j.ijhydene.2016.08.156
Su Q, Gu L, Yao Y et al (2017) Layered double hydroxides derived Nix(MgyAlzOn) catalysts: enhanced ammonia decomposition by hydrogen spillover effect. Appl Catal B 201:451–460. https://doi.org/10.1016/j.apcatb.2016.08.051
Okura K, Miyazaki K, Muroyama H et al (2018) Ammonia decomposition over Ni catalysts supported on perovskite-type oxides for the on-site generation of hydrogen. RSC Adv 8:32102–32110. https://doi.org/10.1039/C8RA06100A
Hansgen DA, Vlachos DG, Chen JG (2010) Using first principles to predict bimetallic catalysts for the ammonia decomposition reaction. Nat Chem 2:484–489. https://doi.org/10.1038/nchem.626
Gu Y, Ma Y, Long Z et al (2021) One-pot synthesis of supported Ni@Al2O3 catalysts with uniform small-sized Ni for hydrogen generation via ammonia decomposition. Int J Hydrogen Energy 46:4045–4054. https://doi.org/10.1016/j.ijhydene.2020.11.003
Wu ZW, Li X, Qin YH et al (2020) Ammonia decomposition over SiO2-supported Ni-Co bimetallic catalyst for COx-free hydrogen generation. Int J Hydrogen Energy 45:15263–15269. https://doi.org/10.1016/j.ijhydene.2020.04.007
Lucentini I, García Colli G, Luzi CD et al (2021) Catalytic ammonia decomposition over Ni-Ru supported on CeO2 for hydrogen production: effect of metal loading and kinetic analysis. Appl Catal B 286:119896. https://doi.org/10.1016/j.apcatb.2021.119896
Li X, Ji W, Zhao J et al (2005) Ammonia decomposition over Ru and Ni catalysts supported on fumed SiO2, MCM-41, and SBA-15. J Catal 236:181–189. https://doi.org/10.1016/j.jcat.2005.09.030
Deng QF, Zhang H, Hou XX et al (2012) High-surface-area Ce0.8Zr0.2O2 solid solutions supported Ni catalysts for ammonia decomposition to hydrogen. Int J Hydrogen Energy 37:15901–15907. https://doi.org/10.1016/j.ijhydene.2012.08.069
Zhang J, Xu H, Li W (2005) Kinetic study of NH3 decomposition over Ni nanoparticles: the role of La promoter, structure sensitivity and compensation effect. Appl Catal A 296:257–267. https://doi.org/10.1016/j.apcata.2005.08.046
Bell TE, Torrente-Murciano L (2016) H2 production via ammonia decomposition using non-noble metal catalysts: a review. Top Catal 59:1438–1457. https://doi.org/10.1007/s11244-016-0653-4
Öcal M, Oukaci R, Marcelin G et al (2000) Steady-state isotopic transient kinetic analysis on Pd-supported hexaaluminates used for methane combustion in the presence and absence of NO. Catal Today 59:205–217. https://doi.org/10.1016/S0920-5861(00)00284-4
Groppi G, Cristiani C, Forzatti P (2001) Preparation, characterisation and catalytic activity of pure and substituted La-hexaaluminate systems for high temperature catalytic combustion. Appl Catal B 35:137–148. https://doi.org/10.1016/S0926-3373(01)00248-X
Ihl Woo S, Kyu Kang S, Min Sohn J (1998) Effect of water content in the precursor solution on the catalytic property and stability of Sr0.8La0.2MnAl11O19 high-temperature combustion catalyst. Appl Catal B 18:317–324. https://doi.org/10.1016/S0926-3373(98)00052-6
Torrez-Herrera JJ, Korili SA, Gil A (2021) Effect of the synthesis method on the morphology, textural properties and catalytic performance of La-hexaaluminates in the dry reforming of methane. J Environ Chem Eng 9:105298. https://doi.org/10.1016/j.jece.2021.105298
Bohre A, Hočevar B, Grilc M et al (2019) Selective catalytic decarboxylation of biomass-derived carboxylic acids to bio-based methacrylic acid over hexaaluminate catalysts. Appl Catal B 256:117889. https://doi.org/10.1016/j.apcatb.2019.117889
Chen F, Chi Y, Zhang H et al (2021) Band-gap shrinked NiO@Co3O4 nanotubes as high-performance supercapacitor electrodes. J Alloy Compd 888:161463. https://doi.org/10.1016/j.jallcom.2021.161463
Ou Z, ZhangQin ZC et al (2021) Highly active and stable Ni/perovskite catalysts in steam methane reforming for hydrogen production. Sustainable Energy Fuels 5:1845–1856. https://doi.org/10.1039/D1SE00082A
Gu YQ, Jin Z, Zhang H et al (2015) Transition metal nanoparticles dispersed in an alumina matrix as active and stable catalysts for COx-free hydrogen production from ammonia. J Mater Chem A 3:17172–17180. https://doi.org/10.1039/C5TA04179A
Okura K, Okanishi T, Muroyama H et al (2015) Promotion effect of rare-earth elements on the catalytic decomposition of ammonia over Ni/Al2O3 catalyst. Appl Catal A 505:77–85. https://doi.org/10.1016/j.apcata.2015.07.020
Choudhary TV, Sivadinarayana C, Goodman DWJCL (2001) Catalytic ammonia decomposition: COx-free hydrogen production for fuel cell applications. Catal Lett 72:197–201. https://doi.org/10.1023/A:1009023825549
Kurtoğlu SF, Sarp S, Yılmaz Akkaya C et al (2018) COx-free hydrogen production from ammonia decomposition over sepiolite-supported nickel catalysts. Int J Hydrogen Energy 43:9954–9968. https://doi.org/10.1016/j.ijhydene.2018.04.057
Meng T, Xu QQ, Li YT et al (2015) Nickle nanoparticles highly dispersed on reduced graphene oxide for ammonia decomposition to hydrogen. J Ind Eng Chem 32:373–379. https://doi.org/10.1016/j.jiec.2015.09.017
Fu E, Qiu Y, Lu H et al (2021) Enhanced NH3 decomposition for H2 production over bimetallic M(M=Co, Fe, Cu)Ni/Al2O3. Fuel Process Technol 221:106945. https://doi.org/10.1016/j.fuproc.2021.106945
Sato K, Abe N, Kawagoe T et al (2017) Supported Ni catalysts prepared from hydrotalcite-like compounds for the production of hydrogen by ammonia decomposition. Int J Hydrogen Energy 42:6610–6617. https://doi.org/10.1016/j.ijhydene.2016.11.150
Nakamura I, Fujitani T (2016) Role of metal oxide supports in NH3 decomposition over Ni catalysts. Appl Catal A: Genera 524:45–49. https://doi.org/10.1016/j.apcata.2016.05.020
Huang C, Li H, Yang J et al (2019) Ce0.6Zr0.3Y0.1O2 solid solutions-supported NiCo bimetal nanocatalysts for NH3 decomposition. Appl Surf Sci 478:708–716. https://doi.org/10.1016/j.apsusc.2019.01.269
Hu ZP, Weng CC, Yuan GG et al (2018) Ni nanoparticles supported on mica for efficient decomposition of ammonia to COx-free hydrogen. Int J Hydrogen Energy 43:9663–9676. https://doi.org/10.1016/j.ijhydene.2018.04.029
Hu ZP, Weng CC, Chen C et al (2018) Catalytic decomposition of ammonia to COx-free hydrogen over Ni/ZSM-5 catalysts: a comparative study of the preparation methods. Appl Catal A 562:49–57. https://doi.org/10.1016/j.apcata.2018.05.038
Yin SF, Xu BQ, Zhou XP et al (2004) A mini-review on ammonia decomposition catalysts for on-site generation of hydrogen for fuel cell applications. Appl Catal A 277:1–9. https://doi.org/10.1016/j.apcata.2004.09.020
Acknowledgements
This work is supported by the National Natural Science Foundation of China (21878001, 22078002 and 22078027). Special thanks to the support from Advanced Catalysis and Green Manufacturing Collaborative Innovation Center, Changzhou University (ACGM2016-06-02 and ACGM2016-06-03), A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflict to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, G., Yu, X., Lei, Z. et al. Preparation of Lanthanum Hexaaluminate Supported Nickel Catalysts for Hydrogen Production by Ammonia Decomposition. Catal Lett 153, 3148–3158 (2023). https://doi.org/10.1007/s10562-022-04214-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04214-w