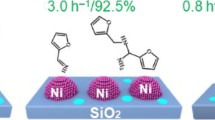
Abstract
Ni catalysts are commonly used for important transformations in organic chemistry. However, they frequently attend the employment as Ni complexes. Herein, we design an efficient and environmentally compatible nanoscale Ni catalyst for debenzylation reactions via hydrogenative C–N bond cleavage. The Ni nanoparticles (NPs) can be in situ generated by pure H2 reduction based on the precursor Ni–Al hydrotalcite-like compound. The Ni nanocatalysts were systematically characterized with various technique approaches including physical adsorption, XRD, Raman, H2-TPR, TEM, and SEM. Two types of Ni species can be detected, i.e., NiO and Ni0. The latter was supposed to be the active sites. The ultra-small and highly dispersed active Ni0 NPs with average diameters of 5 nm can be formed on the surface of the Ni–Al2O3–H2–78 catalyst. Then the complete conversion and high yield (> 90%) for hydrogenative C–N bond cleavage of some substrates (6 examples) can be achieved.
Graphical Abstract









Similar content being viewed by others
References
Wang DY, Kawahata M, Yang ZK, Miyamoto K, Komagawa S, Yamaguchi K, Wang C, Uchiyama M (2016) Stille coupling via C–N bond cleavage. Nat Commun 7:12937–12945
Walker JA Jr, Vickerman KL, Humke JN, Stanley LM (2017) Ni-catalyzed alkene carboacylation via amide C–N bond activation. J Am Chem Soc 139:10228–10231
Ouyang K, Hao W, Zhang WX, Xi Z (2015) Transition-metal-catalyzed cleavage of C–N single bonds. Chem Rev 115:12045–12090
Zhao W, Liu S, Wang H, Yang J, Chen X (2021) Ultrasmall Pd nanoparticles supported on TiO2 for catalytic debenzylation via hydrogenative C–N bond cleavage. ACS Appl Nano Mater 4:159–166
Liu S, Ji F, Li X, Pan X, Chen S, Wang X, Zhang Y, Men Y (2019) Stick-like mesoporous titania loaded Pd as highly active and cost effective catalysts for hydrodebenzylation of hexabenzylhexaazaisowurtzitane (HBIW). Mol Catal 477:110556–110567
Lou D, Wang H, Liu S, Li L, Zhao W, Chen X, Wang J, Li X, Wu P, Yang J (2018) PdFe bimetallic catalysts for debenzylation of hexabenzylhexaazaisowurtzitane (HBIW) and tetraacetyldibenzylhexaazaisowurtzitane (TADBIW). Catal Commun 109:28–32
Zhang M, Liu S, Li L, Li X, Huang H, Yin J, Shao X, Yang J (2017) Effect of carbon supports on Pd catalyst for hydrogenation debenzylation of hexabenzylhexaazaisowurtzitane (HBIW). J Energ Mater 35:251–264
Bailey PD, Beard MA, Dang HPT, Phillips TR, Price RA, Whittaker JH (2008) Debenzylation using catalytic hydrogenolysis in trifluoroethanol, and the total synthesis of (−)-raumacline. Tetrahedron Lett 49:2150–2153
Zhou G, Zhang L, Xue Y, Li J (2019) Progress of N-benzyl removal, Chin. J Org Chem 39:2428–2442
Pandarus V, Beland F, Ciriminna R, Pagliaro M (2011) Selective debenzylation of benzyl protected groups with siliacat Pd(0) under mild conditions. ChemCatChem 3:1146–1150
Mao Y, Liu Y, Hu Y, Wang L, Zhang S, Wang W (2018) Pd-catalyzed debenzylation and deallylation of ethers and esters with sodium hydride. ACS Catal 8:3016–3020
Yakukhnov SA, Ananikov VP (2019) Catalytic transfer hydrodebenzylation with low palladium loading. Adv Synth Catal 361:4781–4789
Moriyama K, Nakamura Y, Togo H (2014) Oxidative debenzylation of N-benzyl amides and O-benzyl ethers using alkali metal bromide. Org Lett 16:3812–3815
Ji H, Jing Q, Huang J, Silverman RB (2012) Acid-facilitated debenzylation of N-Boc, N-benzyl double protected 2-aminopyridinomethyl pyrrolidine derivatives. Tetrahedron 68:1359–1366
Graham TH (2015) Deprotection of N-benzylbenzimidazoles and N-benzylimidazoles with triethylsilane and Pd/C. Tetrahedron Lett 56:2688–2690
Albano G, Evangelisti C, Aronica LA (2017) Hydrogenolysis of benzyl protected phenols and aniline promoted by supported palladium nanoparticles. Chem Sel 2:384–388
Yamamoto Y, Shimizu E, Ban K, Wada Y, Mizusaki T, Yoshimura M, Takagi Y, Sawama Y, Sajiki H (2020) Facile hydrogenative deprotection of N-benzyl groups using a mixed catalyst of palladium and niobic acid-on-carbon. ACS Omega 5:2699–2709
Groppo E, Agostini G, Piovano A, Muddada NB, Leofanti G, Pellegrini R, Portale G, Longo A, Lamberti C (2012) Effect of reduction in liquid phase on the properties and the catalytic activity of Pd/Al2O3 catalysts. J Catal 287:44–54
Perosa A, Tundo P, Zinovyev S (2002) Mild catalytic multiphase hydrogenolysis of benzyl ethers. Green Chem 4:492–494
Hu J, Sun H, Cai W, Pu X, Zhang Y, Shi Z (2016) Nickel-catalyzed borylation of aryl- and benzyltrimethylammonium salts via C–N bond cleavage. J Org Chem 81:14–24
Shi S, Szostak M (2016) Nickel-catalyzed diaryl ketone synthesis by N–C cleavage: direct Negishi cross-coupling of primary amides by site-selective N, N-Di-boc activation. Org Lett 18:5872–5875
Nagae H, Xia J, Kirillov E, Higashida K, Shoji K, Boiteau V, Zhang W, Carpentier JF, Mashima K (2020) Asymmetric allylic alkylation of β-ketoesters via C–N bond cleavage of N-allyl-N-methylaniline derivatives catalyzed by a nickel–diphosphine system. ACS Catal 10:5828–5839
Ritleng V, Henrion M, Chetcuti MJ (2016) Nickel N-heterocyclic carbene-catalyzed C-heteroatom bond formation, reduction, and oxidation: reactions and mechanistic aspects. ACS Catal 6:890–906
Roy D, Sarkar S, Laha RM, Pramanik N, Maiti DK (2015) Ni(0)-Cu(I): a powerful combo catalyst for simultaneous coupling and cleavage of the C–N bond with cyclization to valuable amide-based pyrroles and 4-pyridones. RSC Adv 5:73346–73351
Yang ZK, Xu NX, Takita R, Muranaka A, Wang C, Uchiyama M (2018) Cross-coupling polycondensation via C–O or C–N bond cleavage. Nat Commun 9:1587–1593
Han H, Park S, Jang D, Kim WB (2021) N-doped carbon nanoweb-supported Ni/NiO heterostructure as hybrid catalysts for hydrogen evolution reaction in an alkaline phase. J Alloys Compd 853:157338–157344
Ye X, An Y, Xu G (2011) Kinetics of 9-ethylcarbazole hydrogenation over Raney-Ni catalyst for hydrogen storage. J Alloys Compd 509:152–156
Chen C, Wu D, Liu P, Xia H, Zhou M, Hou X, Jiang J (2021) Efficient Ni-based catalysts for the hydrotreatment of lignin dimer model compounds to cycloalkanes/cycloalkanols. React Chem Eng 6:559–571
Lange S, Formenti D, Lund H, Kreyenschulte C, Agostini G, Bartling S, Bachmann S, Scalone M, Junge K, Beller M (2019) Additive-free nickel-catalyzed debenzylation reactions via hydrogenative C–O and C–N bond cleavage. ACS Sustain Chem Eng 7:17107–17113
He L, Huang Y, Wang A, Wang X, Chen X, Jose Delgado J, Zhang T (2012) A noble-metal-free catalyst derived from Ni-Al hydrotalcite for hydrogen generation from N2H4 center dot H2O decomposition. Angew Chem Int Edit 51:6191–6194
Dong J, Zhu T, Li H, Sun H, Wang Y, Niu L, Wen X, Bai G (2019) Biotemplate-assisted synthesis of layered double oxides confining ultrafine Ni nanoparticles as a stable catalyst for phenol hydrogenation. Ind Eng Chem Res 58:14688–14694
Bravo-Suárez JJ, Páez-Mozo EA, Oyama ST (2004) Review of the synthesis of layered double hydroxides: a thermodynamic approach. Quim Nova 27:601–614
Fu X, Cook JM (1993) General approach for the synthesis of ajmaline-related alkaloids. Enantiospecific total synthesis of (-)-suaveoline, (-)-raumacline, and (-)-Nb-methylraumacline. J Org Chem 58:661–672
Samudrala PS, Nakhate AV, Gupta SSR, Rasal KB, Deshmukh GP, Gadipelly CR, Theegala S, Dumbre DK, Periasamy S, Komandur VRC, Bhargava SK, Mannepalli LK (2019) Oxidative coupling of carboxylic acids or benzaldehydes with DMF using hydrotalicite-derived oxide catalysts. Appl Catal B 240:348–357
Chen H, He S, Xu M, Wei M, Eyans DG, Duan X (2017) Promoted synergic catalysis between metal Ni and acid-base sites toward oxidant-free dehydrogenation of alcohols. ACS Catal 7:2735–2743
Zhu H, Dong H, Laveille P, Saih Y, Caps V, Basset JM (2014) Metal oxides modified NiO catalysts for oxidative dehydrogenation of ethane to ethylene. Catal Today 228:58–64
Chen H, Zhang T, Qian C, Chen X (2010) A novel method for N-alkylation of aliphatic amines with ethers over gamma-Al2O3. Chem Pap 64:537–540
Streng ES, Lee DS, George MW, Poliakoff M (2017) Continuous N-alkylation reactions of amino alcohols using gamma-Al2O3 and supercritical CO2: unexpected formation of cyclic ureas and urethanes by reaction with CO2. Beilstein J Org Chem 13:329–337
Shimizu K, Imaiida N, Kon K, Siddiki SMAH, Satsuma A (2013) Heterogeneous Ni catalysts for N-alkylation of amines with alcohols. ACS Catal 3:998–1005
Charvieux A, Le Moigne L, Borrego LG, Duguet N, Metay E (2019) Solvent-free N-alkylation of amides with alcohols catalyzed by nickel on silica-alumina. Eur J Inorg Chem 2019:6842–6846
Phuoc Hoang H, de Luna GS, Ospitali F, Fornasari G, Vaccari A, Benito P (2020) Open-cell foams coated by Ni/X/Al hydrotalcite-type derived catalysts (X = Ce, La, Y) for CO2 methanation. J CO2 Util. https://doi.org/10.1016/j.jcou.2020.101327
Zhou F, Pan N, Chen H, Xu X, Wang C, Du Y, Guo Y, Zeng Z, Li L (2019) Hydrogen production through steam reforming of toluene over Ce, Zr or Fe promoted Ni–Mg-Al hydrotalcite-derived catalysts at low temperature, Energ Convers. Manage 196:677–687
Acknowledgements
This work is financially supported by the National Natural Science Foundation of China (Grant No. 21503264), a key deployment project from Chinese academy of sciences, the Talent Program of Shanghai University of Engineering Science, Science and Technology Commission of Shanghai Municipality (Grant No. 18030501100).
Funding
National Natural Science Foundation of China, 21503264
Author information
Authors and Affiliations
Contributions
SL: Project administration, Conceptualization, Methodology, Supervision, Formal analysis, Writing-original draft, Writing-review & editing. WZ: Data curation, Investigation, Visualization. ZT: Investigation, Visualization, Validation. LZ: Data curation. KG: Validation. HW: Validation. YM: Conceptualization, Formal analysis. JY: Funding acquisition. JD: Validation.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, S., Zhao, W., Tang, Z. et al. Design of a Nanoscale Ni Catalyst for Debenzylation Reactions via Hydrogenative C–N Bond Cleavage. Catal Lett 153, 3031–3043 (2023). https://doi.org/10.1007/s10562-022-04196-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04196-9