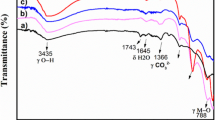
Abstract
An efficient heterogeneous catalyst with 51.63% palladium (Pd) content is synthesized biogenically using mango leaf extract. The phytochemicals present in plant extract are proven to be responsible for the reduction of Pd (II) to Pd (0) without the need of an external reductant. The formation of Pd (0) is confirmed by XRD, UV–Visible analysis. The as-synthesized heterogeneous Pd–NPs catalyst demonstrated a remarkable catalytic activity in the direct C–H arylation of pentafluorobenzene and Hiyama cross coupling. The Pd–NPs were found to be exhibit a superior electrocatalytic activity in the hydrogen evolution reaction (HER). The Pd–NPs catalyst required an over potential − 94 mV to achieve a current density of 10 mA/cm2. Further, the Tafel slope revealed that HER proceeds via Volmer–Heyrovsky mechanism. Additionally, the mechanistic elucidation was addressed and catalyst recyclability was examined and found to be promising.
Graphical Abstract

















Similar content being viewed by others
References
Balanta A, Godard C, Claver C (2011) Pd nanoparticles for C-C coupling reactions. Chem Soc Rev 40(10):4973–4985
Ito Y, Cong W, Fujita T, Tang Z, Chen M (2015) High catalytic activity of nitrogen and sulfur co-doped nanoporous graphene in the hydrogen evolution reaction. Angew Chem 127(7):2159–2164
Narayanan R, El-Sayed MA (2005) Catalysis with transition metal nanoparticles in colloidal solution: nanoparticle shape dependence and stability. J Phys Chem B 109(26):12663–12676
Pacchioni G, Freund HJ (2018) Controlling the charge state of supported nanoparticles in catalysis: lessons from model systems. Chem Soc Rev 47(22):8474–8502
Tukhani M, Panahi F, Khalafi-Nezhad A (2018) Supported palladium on magnetic nanoparticles–starch substrate (Pd-MNPSS): highly efficient magnetic reusable catalyst for C-C coupling reactions in water. ACS Sustain Chem Eng 6(1):1456–1467
Kandathil V, Dateer RB, Sasidhar BS, Patil SA, Patil SA (2018) Green synthesis of palladium nanoparticles: applications in aryl halide cyanation and hiyama cross-coupling reaction under ligand free conditions. Catal Lett 148(6):1562–1578
Manjunatha K, Koley TS, Kandathil V, Dateer RB, Balakrishna G, Sasidhar BS, Patil SA, Patil SA (2018) Magnetic nanoparticle-tethered Schiff base–palladium (II): highly active and reusable heterogeneous catalyst for Suzuki-Miyaura cross-coupling and reduction of nitroarenes in aqueous medium at room temperature. Appl Organomet Chem 32(4):e4266
Nasrollahzadeh M, Sajadi SM, Maham M, Ehsani A (2015) Facile and surfactant-free synthesis of Pd nanoparticles by the extract of the fruits of Piper longum and their catalytic performance for the Sonogashira coupling reaction in water under ligand-and copper-free conditions. RSC Adv 5(4):2562–2567
Flanagan KA, Sullivan JA, Müeller-Bunz H (2007) Preparation and characterization of 4-dimethylaminopyridine-stabilized palladium nanoparticles. Langmuir 23(25):12508–12520
Nemamcha A, Rehspringer JL, Khatmi D (2006) Synthesis of palladium nanoparticles by sonochemical reduction of palladium (II) nitrate in aqueous solution. J Phys Chem B 110(1):383–387
Xiong Y, Chen J, Wiley B, Xia Y, Aloni S, Yin Y (2005) Understanding the role of oxidative etching in the polyol synthesis of Pd nanoparticles with uniform shape and size. J Am Chem Soc 127(20):7332–7333
Cristoforetti G, Pitzalis E, Spiniello R, Ishak R, Muniz-Miranda M (2011) Production of palladium nanoparticles by pulsed laser ablation in water and their characterization. J Phys Chem C 115(12):5073–5083
Czerwosz E, Diduszko R, Dłużewski P, Kęczkowska J, Kozłowski M, Rymarczyk J, Suchańska M (2007) Properties of Pd nanocrystals prepared by PVD method. Vacuum 82(4):372–376
Kim SH, Moon SY, Park JY (2017) Non-colloidal nanocatalysts fabricated using arc plasma deposition and their application in heterogenous catalysis and photocatalysis. Top Catal 60(12):812–822
Pal M, Sasaki T, Koshizaki N (2001) Preparation of Pd/TiO2 nanocomposite by magnetron sputtering. Scr Mater 44(8–9):1817–1820
Byrappa K, Ohara S, Adschiri T (2008) Nanoparticles synthesis using supercritical fluid technology–towards biomedical applications. Adv Drug Deliv Rev 60(3):299–327
Peralta-Videa JR, Zhao L, Lopez-Moreno ML, de la Rosa G, Hong J, Gardea-Torresdey JL (2011) Nanomaterials and the environment: a review for the biennium 2008–2010. J Hazard Mater 186(1):1–5
Hegde RV, Ghosh A, Patil SA, Dateer RB (2019) Pd-nanoparticles catalyzed denitrogenative coupling of aryl halides with arylhydrazines: Greener approach for biaryls synthesis under ligand-free condition. Tetrahedron 75(52):130777
Kandathil V, Kempasiddaiah M, Sasidhar BS, Patil SA (2019) From agriculture residue to catalyst support; a green and sustainable cellulose-based dip catalyst for CC coupling and direct arylation. Carbohydr Polym 1(223):115060
Vishal K, Fahlman BD, Sasidhar BS, Patil SA, Patil SA (2017) Magnetic nanoparticle-supported N-heterocyclic carbene-palladium (II): a convenient, efficient and recyclable catalyst for Suzuki-Miyaura cross-coupling reactions. Catal Lett 147(4):900–918
Khan M, Khan M, Kuniyil M, Adil SF, Al-Warthan A, Alkhathlan HZ, Tremel W, Tahir MN, Siddiqui MR (2014) Biogenic synthesis of palladium nanoparticles using Pulicaria glutinosa extract and their catalytic activity towards the Suzuki coupling reaction. Dalton Trans 43(24):9026–9031
Majumdar R, Tantayanon S, Bag BG (2017) Synthesis of palladium nanoparticles with leaf extract of Chrysophyllum cainito (Star apple) and their applications as efficient catalyst for C-C coupling and reduction reactions. Int Nano Lett 7(4):267–274
Petla RK, Vivekanandhan S, Misra M, Mohanty AK, Satyanarayana N (2011) Soybean (Glycine max) leaf extract based green synthesis of palladium nanoparticles. J Biomater Nanobiotechnol 3.
Sarmah M, Neog AB, Boruah PK, Das MR, Bharali P, Bora U (2019) Effect of substrates on catalytic activity of biogenic palladium nanoparticles in C-C cross-coupling reactions. ACS Omega 4(2):3329–3340
Yang X, Li Q, Wang H, Huang J, Lin L, Wang W, Sun D, Su Y, Opiyo JB, Hong L, Wang Y (2010) Green synthesis of palladium nanoparticles using broth of Cinnamomum camphora leaf. J Nanopart Res 12(5):1589–1598
De Souza CD, Nogueira BR, Rostelato ME (2019) Review of the methodologies used in the synthesis gold nanoparticles by chemical reduction. J Alloys Compd 25(798):714–740
Panigrahi S, Kundu S, Ghosh S, Nath S, Pal T (2004) General method of synthesis for metal nanoparticles. J Nanopart Res 6(4):411–414
Reverberi AP, Kuznetsov NT, Meshalkin VP, Salerno M, Fabiano B (2016) Systematical analysis of chemical methods in metal nanoparticles synthesis. Theor Found Chem Eng 50(1):59–66
Saldan I, Semenyuk Y, Marchuk I, Reshetnyak O (2015) Chemical synthesis and application of palladium nanoparticles. J Mater Sci 50(6):2337–2354
Piermatti O (2021) Green synthesis of Pd nanoparticles for sustainable and environmentally benign processes. Catalysts 11(11):1258
Hegde RV, Ghosh A, Jadhav AH, Nizam A, Patil SA, Peter F, Dateer RB (2021) Biogenic synthesis of Pd-nanoparticles using Areca Nut Husk extract: a greener approach to access α-keto imides and stilbenes. New J Chem 45(35):16213–16222
Amii H, Uneyama K (2009) C− F bond activation in organic synthesis. Chem Rev 109(5):2119–2183
Kasai K, Aoyagi M, Fujita M (2000) Flexible coordination networks with fluorinated backbones. Remarkable ability for induced-fit enclathration of organic molecules. J Am Chem Soc 122(9):2140–2141
Tsuzuki T, Shirasawa N, Suzuki T, Tokito S (2003) Color tunable organic light-emitting diodes using pentafluorophenyl-substituted iridium complexes. Adv Mater 15(17):1455–1458
Zhang J, Chen W, Rojas AJ, Jucov EV, Timofeeva TV, Parker TC, Barlow S, Marder SR (2013) Controllable direct arylation: fast route to symmetrical and unsymmetrical 4, 7-diaryl-5, 6-difluoro-2, 1, 3-benzothiadiazole derivatives for organic optoelectronic materials. J Am Chem Soc 135(44):16376–16379
Lai YC, Chen HY, Hung WC, Lin CC, Hong FE (2005) Palladium catalyzed Suzuki cross-coupling reactions using N. O-bidentate ligands Tetrahedron 61(40):9484–9489
Liu L, Zhang Y, Xin B (2006) Synthesis of biaryls and polyaryls by ligand-free Suzuki reaction in aqueous phase. J Org Chem 71(10):3994–3997
Liu Z, Luan N, Shen L, Li J, Zou D, Wu Y, Wu Y (2019) Palladium-catalyzed hiyama cross-couplings of arylsilanes with 3-iodoazetidine: synthesis of 3-arylazetidines. J Org Chem 84(19):12358–12365
Schroeter F, Soellner J, Strassner T (2018) Cyclometalated palladium NHC complexes bearing PEG chains for Suzuki-Miyaura cross-coupling in water. Organometallics 37(22):4267–4275
So CM, Lau CP, Kwong FY (2008) A general palladium-catalyzed suzuki-miyaura coupling of aryl mesylates. Angew Chem 120(42):8179–8183
Yang J, Wang L (2012) Synthesis and characterization of dinuclear NHC–palladium complexes and their applications in the Hiyama reactions of aryltrialkyoxysilanes with aryl chlorides. Dalton Trans 41(39):12031–12037
Bernhammer JC, Huynh HV (2012) Pyrazolin-5-ylidene palladium (II) complexes: Synthesis, characterization, and application in the direct arylation of pentafluorobenzene. Organometallics 31(14):5121–5130
Chang JW, Chia EY, Chai CL, Seayad J (2012) Scope of direct arylation of fluorinated aromatics with aryl sulfonates. Org Biomol Chem 10(11):2289–2299
Chen F, Min QQ, Zhang X (2012) Pd-catalyzed direct arylation of polyfluoroarenes on water under mild conditions using PPh3 ligand. J Org Chem 77(6):2992–2998
Lafrance M, Rowley CN, Woo TK, Fagnou K (2006) Catalytic intermolecular direct arylation of perfluorobenzenes. J Am Chem Soc 128(27):8754–8756
Yuan D, Huynh HV (2012) 1, 2, 3-Triazolin-5-ylidenes: synthesis of hetero-bis (carbene) Pd (II) complexes, determination of donor strengths, and catalysis. Organometallics 31(1):405–412
Bernsmeier D, Sachse R, Bernicke M, Schmack R, Kettemann F, Polte J, Kraehnert R (2019) Outstanding hydrogen evolution performance of supported Pt nanoparticles: incorporation of preformed colloids into mesoporous carbon films. J Catal 1(369):181–189
Durst J, Simon C, Hasché F, Gasteiger HA (2014) Hydrogen oxidation and evolution reaction kinetics on carbon supported Pt, Ir, Rh, and Pd electrocatalysts in acidic media. J Electrochem Soc 162(1):F190
Grigoriev SA, Millet P, Fateev VN (2008) Evaluation of carbon-supported Pt and Pd nanoparticles for the hydrogen evolution reaction in PEM water electrolysers. J Power Sources 177(2):281–285
Ojani R, Valiollahi R, Raoof JB (2014) Comparison between graphene supported Pt hollow nanospheres and graphene supported Pt solid nanoparticles for hydrogen evolution reaction. Energy 1(74):871–876
Xiao Y, Wang W, Li T, Mao Y, Liu C (2021) Onion-like core-shell Ni@ C supported on carbon nanotubes decorated with low Pt as a superior electrocatalyst for hydrogen evolution reaction. Electrochim Acta 1(386):138406
Sarkar S, Peter SC (2018) An overview on Pd-based electrocatalysts for the hydrogen evolution reaction. Inorg Chem Front 5(9):2060–2080
Gerlitz I, Fiegenbaum-Raz M, Bar-Sadan M, Cohen H, Ismach A, Rosen BA (2020) Catalytic hydrogen evolution reaction enhancement on vertically aligned MoS2 by synergistic addition of silver and palladium. ChemElectroChem 7(20):4224–4232
Ghasemi S, Hosseini SR, Nabipour S, Asen P (2015) Palladium nanoparticles supported on graphene as an efficient electrocatalyst for hydrogen evolution reaction. Int J Hydrogen Energy 40(46):16184–16191
Karthick K, Bijoy TK, Sivakumaran A, Mansoor Basha AB, Murugan P, Kundu S (2020) Enhancing hydrogen evolution reaction activities of 2H-phase VS2 layers with palladium nanoparticles. Inorg Chem 59(14):10197–10207
Nguyen-Ba K, Vargas-García JR, Manzo-Robledo A (2020) Alternative synthesis of structurally defective MoS2 nanoflakes for efficient hydrogen evolution reaction. Mater Sci Eng, B 1(256):114539
Woldetinsay M, Refera T, Olu F, Maiyalagan T (2020) Synergetic effect between MoS2 and N, S-doped reduced graphene oxide supported palladium nanoparticles for hydrogen evolution reaction. Mater Chem Phys 1(251):123106
Xu W, Zhu S, Liang Y, Cui Z, Yang X, Inoue A, Wang H (2017) A highly efficient electrocatalyst based on amorphous Pd–Cu–S material for hydrogen evolution reaction. J Mater Chem A 5(35):18793–18800
Zhang L, Chang Q, Chen H, Shao M (2016) Recent advances in palladium-based electrocatalysts for fuel cell reactions and hydrogen evolution reaction. Nano Energy 1(29):198–219
Cai J, Javed R, Ye D, Zhao H, Zhang J (2020) Recent progress in noble metal nanocluster and single atom electrocatalysts for the hydrogen evolution reaction. J Mater Chem A 8(43):22467–22487
Chang L, Sun Z, Hu YH (2021) 1T phase transition metal dichalcogenides for hydrogen evolution reaction. Electrochem Energy Rev 4(2):194–218
Kang Z, Khan MA, Gong Y, Javed R, Xu Y, Ye D, Zhao H, Zhang J (2021) Recent progress of MXenes and MXene-based nanomaterials for the electrocatalytic hydrogen evolution reaction. J Mater Chem A 9(10):6089–6108
Wu H, Feng C, Zhang L, Zhang J, Wilkinson DP (2021) Non-noble metal electrocatalysts for the hydrogen evolution reaction in water electrolysis. Electrochem Energy Rev 4(3):473–507
Doucet H, Hierso JC (2007) Palladium-based catalytic systems for the synthesis of conjugated enynes by Sonogashira reactions and related alkynylations. Angew Chem Int Ed 46(6):834–871
Miyaura N, Suzuki A (1995) Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem Rev 95(7):2457–2483
Abdoli M, Mirjafary Z, Saeidian H, Kakanejadifard A (2015) New developments in direct functionalization of C-H and N–H bonds of purine bases via metal catalyzed cross-coupling reactions. RSC Adv 5(55):44371–44389
Beletskaya IP, Ananikov VP (2011) Transition-metal-catalyzed C− S, C− Se, and C− Te bond formation via cross-coupling and atom-economic addition reactions. Chem Rev 111(3):1596–1636
Tappe FM, Trepohl VT, Oestreich M (2010) Transition-metal-catalyzed CP cross-coupling reactions. Synthesis 2010(18):3037–3062
Lee DS, Choy PY, So CM, Wang J, Lau CP, Kwong FY (2012) Palladium-catalyzed direct arylation of polyfluoroarenes with aryl tosylates and mesylates. RSC Adv 2(24):9179–9182
Lei J, Guo C, Liu F, Chen S, Shi WJ, Wang Z, Zhai Z, Mo S, Wang J (2019) Enhancement of electro-optic properties of nonlinear optical chromophores by introducing pentafluorobenzene group into the donor and π-bridge. Dyes Pigm 1(170):107607
Mao S, Shi X, Soulé JF, Doucet H (2019) Pd/C as heterogeneous catalyst for the direct arylation of (poly) fluorobenzenes. Chemistry 25(40):9504–9513
Alacid E, Nájera C (2006) Solvent-less and fluoride-free hiyama reaction of arylsiloxanes with aryl bromides and chlorides promoted by sodium hydroxide: a useful protocol for palladium recycling and product isolation. Adv Synth Catal 348(7–8):945–952
Gordillo A, de Jesús E, López-Mardomingo C (2006) C−C coupling reactions of aryl bromides and arylsiloxanes in water catalyzed by Palladium complexes of phosphanes modified with crown ethers. Org Lett 8(16):3517–3520
Murata M, Shimazaki R, Watanabe S, Masuda Y (2001) Palladium-catalyzed cross-coupling reaction of aryltriethoxysilanes with aryl bromides under basic aqueous conditions. Synthesis 2001(15):2231–2233
Lee DH, Jung JY, Jin MJ (2010) General and highly active catalyst for mono and double Hiyama coupling reactions of unreactive aryl chlorides in water. Chem Commun 46(47):9046–9048
Sakon A, Ii R, Hamasaka G, Uozumi Y, Shinagawa T, Shimomura O, Nomura R, Ohtaka A (2017) Detailed mechanism for Hiyama coupling reaction in water catalyzed by linear polystyrene-stabilized PdO nanoparticles. Organometallics 36(8):1618–1622
Sobhani S, Habibollahi A, Zeraatkar Z (2019) A novel water-dispersible/magnetically recyclable Pd catalyst for C-C cross-coupling reactions in pure water. Org Process Res Dev 23(7):1321–1332
Acknowledgements
Authors would like to acknowledge the support of the Deputyship for Research and Innovation- Ministry of Education, Kingdom of Saudi Arabia for this research through grant (NU/IFC/INT/01/002) under the Institutional Funding Committee at Najran University, Kingdon of Saudi Arabia.The authors thank, DST-SERB, Government of India, for the financial support through the research grant: File Nos. SB/S2/RJN-042/2017 and ECR/2017/002207. Author also thanks to Jain University Start-up Research Grant, for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests concerning this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Limaye, A.S., Alsaiari, M., Shinde, P.V. et al. Greener Approach for Pd–NPs Synthesis Using Mangifera Indica Leaf Extract: Heterogeneous Nano Catalyst for Direct C–H Arylation of (Poly)Fluorobenzene, Hiyama Coupling Reaction and Hydrogen Evolution Reaction Study. Catal Lett 153, 1988–2004 (2023). https://doi.org/10.1007/s10562-022-04138-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04138-5