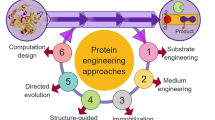
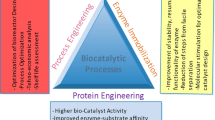
Abstract
Enzymes have replaced traditional industrial catalysts as more efficient, eco-friendly, and sustainable alternatives that can be used in different biotechnological processes, food, and pharmaceutical industries. Yet, the enzymes from nature are engineered to make them adapt and enhance their durability in the industrial environment. Techniques have been developed to tailor such enzymes and overcome the hurdles of efficient bio-catalysis. Protein engineering has transformed the art of enzyme tailoring by providing opportunities to create enzymes with better functionality, such as increased stability, reaction product inhibition, and improved catalytic activity. Protein engineering and immobilization are compatible approaches used side by side to improve enzyme properties. The surge in enzyme immobilization has enabled robustness and outstanding functionality in harsh industrial conditions with high temperatures and organic solvents. The introduction of multi-enzyme catalytic cascades according to a mix of biocatalysts opens up many new possibilities in biosynthesis, biocatalysis, and biotransformation. Multi-enzyme cascade reactions often provide reaction time and cost-related advantages. Immobilization techniques and multi enzymes cascades are used to obtain robust industrial catalysts. This review focuses on the state-of-the-art strategies and trends to construct novel biocatalysts having amplified catalytic activity and substrate specificity required for industrial application. Further development in protein engineering, immobilization techniques, and multi-enzyme cascade reactions might pave the way for future industrial biocatalysis.
Graphical Abstract





Similar content being viewed by others
References
Globe Newswire (2019). Global industrial enzymes market growth, trends, and forecast 2019–2024: competition for raw materials with other industries and price volatility restraining market growth. Available online at: https://www.globenewswire.com/news-release/2019/03/29/1788385/0/en/Global-Industrial-EnzymesMarket-Growth-Trends-and-Forecast-2019-2024-Competition-for-Raw-Materials-with-Other-Industries-and-Price-Volatility-Restraining-Market-Growth.html [Last accessed: July 23, 2022].
Global market insight (2019). Enzymes market. Available online at: https://www.gminsights.com/industry-analysis/enzymes-market [Last accessed: July 23, 2022].
France SP, Hepworth LJ, Turner NJ, Flitsch SL (2017) Constructing biocatalytic cascades: in vitro and in vivo approaches to de novo multi-enzyme pathways. ACS Catal 7(1):710–724
Bilal M, Iqbal H (2019) Tailoring multipurpose biocatalysts via protein engineering approaches: a review. Catal Lett 149(8):2204–2217
Parra-Saldivar R, Ramirez-Mendoza RA, Sharma A, Oza G, Zavala-Yoe R, Iqbal HM (2020) Robust enzymes designing for efficient biocatalysis. In Biomass, Biofuels, Biochemicals (pp. 49–63). Elsevier.
Bilal M, Nguyen TA, Iqbal HM (2020) Multifunctional carbon nanotubes and their derived nano-constructs for enzyme immobilization–a paradigm shift in biocatalyst design. Coord Chem Rev 1(422):213475
Bilal M, Iqbal HM (2021) Armoring bio-catalysis via structural and functional coordination between nanostructured materials and lipases for tailored applications. Int J Biol Macromol 1(166):818–838
Bilal M, Qamar SA, Ashraf SS, Rodríguez-Couto S, Iqbal HM (2021) Robust nanocarriers to engineer nanobiocatalysts for bioprocessing applications. Adv Coll Interface Sci 1(293):102438
Thomas SM, DiCosimo R, Nagarajan V (2002) Biocatalysis: applications and potentials for the chemical industry. Trends Biotechnol 20(6):238–242
Truppo MD (2017) Biocatalysis in the pharmaceutical industry: the need for speed. ACS Med Chem Lett 8(5):476–480
Bilal M, Iqbal HM (2019) Chemical, physical, and biological coordination: an interplay between materials and enzymes as potential platforms for immobilization. Coord Chem Rev 1(388):1–23
Wu S, Snajdrova R, Moore JC, Baldenius K, Bornscheuer UT (2021) Biocatalysis: enzymatic synthesis for industrial applications. Angew Chem Int Ed 60(1):88–119
Aslam S, Ali A, Asgher M, Farah N, Iqbal H, Bilal M (2022) Fabrication and catalytic characterization of laccase-loaded calcium-alginate beads for enhanced degradation of dye-contaminated aqueous solutions. Catal Lett 152(6):1729–1741
Liu L, Qamar SA, Bilal M, Iqbal H (2022) Broadening the catalytic role of enzymes in cosmeceutical sector: a robust tool from white biotechnology. Catal Lett 152(3):707–719
Lopez-Cantu DO, González-González RB, Melchor-Martínez EM, Martínez SA, Araújo RG, Parra-Arroyo L, Sosa-Hernández JE, Parra-Saldívar R, Iqbal HM (2022) Enzyme-mimicking capacities of carbon-dots nanozymes: properties, catalytic mechanism, and applications–a review. Int J Biol Macromol 1(194):676–687
Shahzad MW, Burhan M, Ang L, Ng KC (2017) Energy-water-environment nexus underpinning future desalination sustainability. Desalination 1(413):52–64
Sahoo PC, Kumar M, Puri SK, Ramakumar SS (2018) Enzyme inspired complexes for industrial CO2 capture: opportunities and challenges. J CO2 Utilization 24:419–429
Bilal M, Asgher M, Cheng H, Yan Y, Iqbal HM (2019) Multi-point enzyme immobilization, surface chemistry, and novel platforms: a paradigm shift in biocatalyst design. Crit Rev Biotechnol 39(2):202–219
Alvarado-Ramírez L, Rostro-Alanis M, Rodríguez-Rodríguez J, Sosa-Hernández JE, Melchor-Martínez EM, Iqbal HM, Parra-Saldívar R (2021) enzyme (single and multiple) and nanozyme biosensors: recent developments and their novel applications in the water-food-health nexus. Biosensors 11(11):410
Bilal M, Hussain N, Américo-Pinheiro JH, Almulaiky YQ, Iqbal HM (2021) Multi-enzyme co-immobilized nano-assemblies: Bringing enzymes together for expanding bio-catalysis scope to meet biotechnological challenges. Int J Biol Macromol 1(186):735–749
Bilal M, Cui J, Iqbal HM (2019) Tailoring enzyme microenvironment: State-of-the-art strategy to fulfill the quest for efficient bio-catalysis. Int J Biol Macromol 1(130):186–196
Murata H, Cummings CS, Koepsel RR, Russell AJ (2014) Rational tailoring of substrate and inhibitor affinity via ATRP polymer-based protein engineering. Biomacromol 15(7):2817–2823
Shi J, Wu Y, Zhang S, Tian Y, Yang D, Jiang Z (2018) Bioinspired construction of multi-enzyme catalytic systems. Chem Soc Rev 47(12):4295–4313
Zhang Y, Wang Q, Hess H (2017) Increasing enzyme cascade throughput by pH-engineering the microenvironment of individual enzymes. ACS Catal 7(3):2047–2051
Tang Z, Jin W, Sun R, Liao Y, Zhen T, Chen H, Wu Q, Gou L, Li C (2018) Improved thermostability and enzyme activity of a recombinant phyA mutant phytase from Aspergillus niger N25 by directed evolution and site-directed mutagenesis. Enzyme Microb Technol 1(108):74–81
Dissanayake T, Swails JM, Harris ME, Roitberg AE, York DM (2015) Interpretation of pH–activity profiles for acid–base catalysis from molecular simulations. Biochemistry 54(6):1307–1313
Klitzing R, Moehwald H (1995) Proton concentration profile in ultrathin polyelectrolyte films. Langmuir 11(9):3554–3559
Cobo I, Li M, Sumerlin BS, Perrier S (2015) Smart hybrid materials by conjugation of responsive polymers to biomacromolecules. Nat Mater 14(2):143–159
Lancaster L, Abdallah W, Banta S, Wheeldon I (2018) Engineering enzyme microenvironments for enhanced biocatalysis. Chem Soc Rev 47(14):5177–5186
Gao Y, Roberts CC, Zhu J, Lin JL, Chang CE, Wheeldon I (2015) Tuning enzyme kinetics through designed intermolecular interactions far from the active site. ACS Catal 5(4):2149–2153
Gao Y, Roberts CC, Toop A, Chang CE, Wheeldon I (2016) Mechanisms of enhanced catalysis in enzyme–DNA nanostructures revealed through molecular simulations and experimental analysis. ChemBioChem 17(15):1430–1436
Fu J, Yang YR, Johnson-Buck A, Liu M, Liu Y, Walter NG, Woodbury NW, Yan H (2014) Multi-enzyme complexes on DNA scaffolds capable of substrate channelling with an artificial swinging arm. Nat Nanotechnol 9(7):531–536
Fu J, Yang YR, Dhakal S, Zhao Z, Liu M, Zhang T, Walter NG, Yan H (2016) Assembly of multienzyme complexes on DNA nanostructures. Nat Protoc 11(11):2243–2273
Perham RN (2000) Swinging arms and swinging domains in multifunctional enzymes: catalytic machines for multistep reactions. Annu Rev Biochem 69:961
Liu Y, Du J, Yan M, Lau MY, Hu J, Han H, Yang OO, Liang S, Wei W, Wang H, Li J (2013) Biomimetic enzyme nanocomplexes and their use as antidotes and preventive measures for alcohol intoxication. Nat Nanotechnol 8(3):187–192
Sang LC, Coppens MO (2011) Effects of surface curvature and surface chemistry on the structure and activity of proteins adsorbed in nanopores. Phys Chem Chem Phys 13(14):6689–6698
Bilal M, Zhao Y, Noreen S, Shah SZ, Bharagava RN, Iqbal HM (2019) Modifying bio-catalytic properties of enzymes for efficient biocatalysis: A review from immobilization strategies viewpoint. Biocatal Biotransform 37(3):159–182
Alvarado-Ramírez L, Rostro-Alanis M, Rodríguez-Rodríguez J, Castillo-Zacarías C, Sosa-Hernández JE, Barceló D, Iqbal HM, Parra-Saldívar R (2021) Exploring current tendencies in techniques and materials for immobilization of laccases–a review. Int J Biol Macromol 30(181):683–696
Liu S, Bilal M, Rizwan K, Gul I, Rasheed T, Iqbal HM (2021) Smart chemistry of enzyme immobilization using various support matrices–a review. Int J Biol Macromol 1(190):396–408
Shahbaz A, Hussain N, Intisar A, Bilal M, Iqbal H (2021) Immobilized enzymes-based biosensing cues for strengthening biocatalysis and biorecognition. Catal Lett 27:1–3
Collins J, Zhang T, Oh SW, Maloney R, Fu J (2017) DNA-crowded enzyme complexes with enhanced activities and stabilities. Chem Commun 53(97):13059–13062
Zhao Z, Fu J, Dhakal S, Johnson-Buck A, Liu M, Zhang T, Woodbury NW, Liu Y, Walter NG, Yan H (2016) Nanocaged enzymes with enhanced catalytic activity and increased stability against protease digestion. Nat Commun 7(1):1–9
Goldberg AL (2007) Functions of the proteasome: from protein degradation and immune surveillance to cancer therapy. Biochem Soc Trans 35(1):12–17
Pogson M, Georgiou G, Iverson BL (2009) Engineering next generation proteases. Curr Opin Biotechnol 20(4):390–397
Ladenstein R, Fischer M, Bacher A (2013) The lumazine synthase/riboflavin synthase complex: shapes and functions of a highly variable enzyme system. FEBS J 280(11):2537–2563
Zschoche R, Hilvert D (2015) Diffusion-limited cargo loading of an engineered protein container. J Am Chem Soc 137(51):16121–16132
Frey R, Mantri S, Rocca M, Hilvert D (2016) Bottom-up construction of a primordial carboxysome mimic. J Am Chem Soc 138(32):10072–10075
Bautista-Barrufet A, López-Gallego F, Rojas-Cervellera V, Rovira C, Pericàs MA, Guisán JM, Gorostiza P (2014) Optical control of enzyme enantioselectivity in solid phase. ACS Catal 4(3):1004–1009
Tanaka SI, Takahashi T, Koide A, Iwamoto R, Koikeda S, Koide S (2018) Monobody-mediated alteration of lipase substrate specificity. ACS Chem Biol 13(6):1487–1492
Stein V, Alexandrov K (2015) Synthetic protein switches: design principles and applications. Trends Biotechnol 33(2):101–110
Abdallah W, Solanki K, Banta S (2018) Insertion of a calcium-responsive β-roll domain into a thermostable alcohol dehydrogenase enables tunable control over cofactor selectivity. ACS Catal 8(2):1602–1613
Xin L, Zhou C, Yang Z, Liu D (2013) Regulation of an enzyme cascade reaction by a DNA machine. Small 9(18):3088–3091
Cao L (2005) Immobilised enzymes: science or art? Curr Opin Chem Biol 9(2):217–226
Bilal M, Iqbal HM, Guo S, Hu H, Wang W, Zhang X (2018) State-of-the-art protein engineering approaches using biological macromolecules: A review from immobilization to implementation view point. Int J Biol Macromol 1(108):893–901
Rojas MJ, Amaral-Fonseca M, Zanin GM, Fernandez-Lafuente R, Giordano RD, Tardioli PW (2019) Preparation of crosslinked enzyme aggregates of a thermostable cyclodextrin glucosyltransferase from Thermoanaerobacter sp. critical effect of the crosslinking agent. Catalysts 9(2):120
Cui JD, Sun LM, Li LL (2013) A simple technique of preparing stable CLEAs of phenylalanine ammonia lyase using co-aggregation with starch and bovine serum albumin. Appl Biochem Biotechnol 170(8):1827–1837
Özacar M, Mehde AA, Mehdi WA, Özacar ZZ, Severgün O (2019) The novel multi cross-linked enzyme aggregates of protease, lipase, and catalase production from the sunflower seeds, characterization and application. Colloids Surf, B 1(173):58–68
Fathali Z, Rezaei S, Faramarzi MA, Habibi-Rezaei M (2019) Catalytic phenol removal using entrapped cross-linked laccase aggregates. Int J Biol Macromol 1(122):359–366
Liao Q, Du X, Jiang W, Tong Y, Zhao Z, Fang R, Feng J, Tang L (2018) Cross-linked enzyme aggregates (CLEAs) of halohydrin dehalogenase from Agrobacterium radiobacter AD1: Preparation, characterization and application as a biocatalyst. J Biotechnol 20(272):48–55
Graebin NG, de Andrades D, Barsé LQ, Rodrigues RC, Ayub MA (2018) Preparation and characterization of cross-linked enzyme aggregates of dextransucrase from Leuconostoc mesenteroides B-512F. Process Biochem 1(71):101–108
Irfan M, Gonzalez CF, Raza S, Rafiq M, Hasan F, Khan S, Shah AA (2018) Improvement in thermostability of xylanase from Geobacillus thermodenitrificans C5 by site directed mutagenesis. Enzyme Microb Technol 1(111):38–47
Bedade DK, Muley AB, Singhal RS (2019) Magnetic cross-linked enzyme aggregates of acrylamidase from Cupriavidus oxalaticus ICTDB921 for biodegradation of acrylamide from industrial waste water. Biores Technol 1(272):137–145
Asgher M, Bashir F, Iqbal HM (2018) Protease-based cross-linked enzyme aggregates with improved catalytic stability, silver removal, and dehairing potentials. Int J Biol Macromol 15(118):1247–1256
Kołodziejczak-Radzimska A, Nghiem LD, Jesionowski T (2021) Functionalized materials as a versatile platform for enzyme immobilization in wastewater treatment. Current Pollution Reports 7(3):263–276
Cui JD, Cui LL, Zhang SP, Zhang YF, Su ZG, Ma GH (2014) Hybrid magnetic cross-linked enzyme aggregates of phenylalanine ammonia lyase from Rhodotorula glutinis. PLoS ONE 9(5):e97221
Park JM, Kim M, Park HS, Jang A, Min J, Kim YH (2013) Immobilization of lysozyme-CLEA onto electrospun chitosan nanofiber for effective antibacterial applications. Int J Biol Macromol 1(54):37–43
Hwang ET, Lee B, Zhang M, Jun SH, Shim J, Lee J, Kim J, Gu MB (2012) Immobilization and stabilization of subtilisin Carlsberg in magnetically-separable mesoporous silica for transesterification in an organic solvent. Green Chem 14(7):1884–1887
Wang M, Qi W, Yu Q, Su R, He Z (2010) Cross-linking enzyme aggregates in the macropores of silica gel: a practical and efficient method for enzyme stabilization. Biochem Eng J 52(2–3):168–174
Cui J, Jia S, Liang L, Zhao Y, Feng Y (2015) Mesoporous CLEAs-silica composite microparticles with high activity and enhanced stability. Sci Rep 5(1):1–3
Sheldon RA, Woodley JM (2018) Role of biocatalysis in sustainable chemistry. Chem Rev 118(2):801–838
Periyasamy K, Santhalembi L, Mortha G, Aurousseau M, Subramanian S (2016) Carrier-free co-immobilization of xylanase, cellulase and β-1, 3-glucanase as combined cross-linked enzyme aggregates (combi-CLEAs) for one-pot saccharification of sugarcane bagasse. RSC Adv 6(39):32849–32857
Su E, Meng Y, Ning C, Ma X, Deng S (2018) Magnetic combined cross-linked enzyme aggregates (Combi-CLEAs) for cofactor regeneration in the synthesis of chiral alcohol. J Biotechnol 10(271):1–7
Scism RA, Bachmann BO (2010) Five-component cascade synthesis of nucleotide analogues in an engineered self-immobilized enzyme aggregate. ChemBioChem 11(1):67–70
Shah S, Solanki K, Gupta MN (2007) Enhancement of lipase activity in non-aqueous media upon immobilization on multi-walled carbon nanotubes. Chem Cent J 1(1):1–6
Mahmod SS, Yusof F, Jami MS, Khanahmadi S, Shah H (2015) Development of an immobilized biocatalyst with lipase and protease activities as a multipurpose cross-linked enzyme aggregate (multi-CLEA). Process Biochem 50(12):2144–2157
Bornscheuer UT, Huisman GW, Kazlauskas RJ, Lutz S, Moore JC, Robins K (2012) Engineering the third wave of biocatalysis. Nature 485(7397):185–194
Bell SG, Yang W, Dale A, Zhou W, Wong LL (2013) Improving the affinity and activity of CYP101D2 for hydrophobic substrates. Appl Microbiol Biotechnol 97(9):3979–3990
Chen X, Christopher A, Jones JP, Bell SG, Guo Q, Xu F, Rao Z, Wong LL (2002) Crystal Structure of the F87W/Y96F/V247L mutant of cytochrome P-450cam with 1, 3, 5-trichlorobenzene bound and further protein engineering for the oxidation of pentachlorobenzene and hexachlorobenzene* 210. J Biol Chem 277(40):37519–37526
Wang Y, San KY, Bennett GN (2013) Cofactor engineering for advancing chemical biotechnology. Curr Opin Biotechnol 24(6):994–999
Hasegawa S, Uematsu K, Natsuma Y, Suda M, Hiraga K, Jojima T, Inui M, Yukawa H (2012) Improvement of the redox balance increases L-valine production by Corynebacterium glutamicum under oxygen deprivation conditions. Appl Environ Microbiol 78(3):865–875
Zhang K, Li H, Cho KM, Liao JC (2010) Expanding metabolism for total biosynthesis of the nonnatural amino acid L-homoalanine. Proc Natl Acad Sci 107(14):6234–6239
Agapakis CM, Boyle PM, Silver PA (2012) Natural strategies for the spatial optimization of metabolism in synthetic biology. Nat Chem Biol 8(6):527–535
Besse P, Combourieu B, Poupin P, Sancelme M, Truffaut N, Veschambre H, Delort AM (1998) Degradation of morpholine and thiomorpholine by an environmental mycobacterium involves a cytochrome P450: direct evidence of intermediates by in situ 1H NMR. J Mol Cataly B 5(1–4):403–409
Albertsen L, Chen Y, Bach LS, Rattleff S, Maury J, Brix S, Nielsen J, Mortensen UH (2011) Diversion of flux toward sesquiterpene production in Saccharomyces cerevisiae by fusion of host and heterologous enzymes. Appl Environ Microbiol 77(3):1033–1040
Dueber JE, Wu GC, Malmirchegini GR, Moon TS, Petzold CJ, Ullal AV, Prather KL, Keasling JD (2009) Synthetic protein scaffolds provide modular control over metabolic flux. Nat Biotechnol 27(8):753–759
Sánchez-Moreno I, Bordes I, Castillo R, Ruiz-Pernía JJ, Moliner V, García-Junceda E (2015) Tuning the phosphoryl donor specificity of dihydroxyacetone kinase from ATP to inorganic polyphosphate: an insight from computational studies. Int J Mol Sci 16(11):27835–27849
Zou Z, Mate DM, Rübsam K, Jakob F, Schwaneberg U (2018) Sortase-mediated high-throughput screening platform for directed enzyme evolution. ACS Comb Sci 20(4):203–211
Zhou P, Li M, Shen B, Yao Z, Bian Q, Ye L, Yu H (2019) Directed coevolution of β-carotene ketolase and hydroxylase and its application in temperature-regulated biosynthesis of astaxanthin. J Agric Food Chem 67(4):1072–1080
Varriale S, Cerullo G, Antonopoulou I, Christakopoulos P, Rova U, Tron T, Fauré R, Jütten P, Piechot A, Brás JL, Fontes CM (2018) Evolution of the feruloyl esterase MtFae1a from Myceliophthora thermophila towards improved catalysts for antioxidants synthesis. Appl Microbiol Biotechnol 102(12):5185–5196
Gordon SR, Stanley EJ, Wolf S, Toland A, Wu SJ, Hadidi D, Mills JH, Baker D, Pultz IS, Siegel JB (2012) Computational design of an α-gliadin peptidase. J Am Chem Soc 134(50):20513–20520
Bernal C, Rodriguez K, Martinez R (2018) Integrating enzyme immobilization and protein engineering: an alternative path for the development of novel and improved industrial biocatalysts. Biotechnol Adv 36(5):1470–1480
Illanes A, Cauerhff A, Wilson L, Castro GR (2012) Recent trends in biocatalysis engineering. Biores Technol 1(115):48–57
Schöll A, Kilian L, Zou Y, Ziroff J, Hame S, Reinert F, Umbach E, Fink RH (2010) Disordering of an organic overlayer on a metal surface upon cooling. Science 329(5989):303–305
Hauer B, Branneby CK, Hult K, Magnusson A, Hamberg A, inventors; BASF SE, assignee (2014) Enzymatically catalyzed method of preparing mono-acylated polyols. United States patent US 8,715,970. 2014 May 6.
Godoy CA, Rivas BD, Grazú V, Montes T, Guisán JM, López-Gallego F (2011) Glyoxyl-disulfide agarose: a tailor-made support for site-directed rigidification of proteins. Biomacromol 12(5):1800–1809
Stark MB, Holmberg K (1989) Covalent immobilization of lipase in organic solvents. Biotechnol Bioeng 34(7):942–950
Vahidi AK, Yang Y, Ngo TP, Li Z (2015) Simple and efficient immobilization of extracellular his-tagged enzyme directly from cell culture supernatant as active and recyclable nanobiocatalyst: high-performance production of biodiesel from waste grease. ACS Catal 5(6):3157–3161
Acknowledgements
Consejo Nacional de Ciencia y Tecnología (CONACyT) Mexico is thankfully acknowledged for partially supporting this work under Sistema Nacional de Investigadores (SNI) program awarded to Hafiz M. N. Iqbal (CVU: 735340).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The author(s) declare no conflicting interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Riaz, R., Ashraf, M., Hussain, N. et al. Redesigning Robust Biocatalysts by Engineering Enzyme Microenvironment and Enzyme Immobilization. Catal Lett 153, 1587–1601 (2023). https://doi.org/10.1007/s10562-022-04137-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04137-6