Abstract
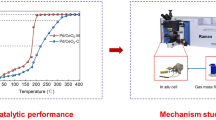
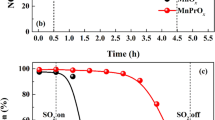
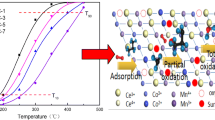
The methanol oxidation reaction is a promising route to eliminating trace amount of methanol in exhaust gases which aroused serious environmental concern. In this work, a novel Pd/MgAl2O4 catalyst was prepared to construct the metal-support interface and employed in the methanol oxidation reaction. The reaction results show that the Pd/MgAl2O4 catalyst could achieve 100% methanol oxidation at 198 ℃ over the Pd/MgO and Pd/Al2O3 catalysts. The high-resolution transmission electron microscopy (HRTEM), X-ray diffraction (XRD), CO-chemisorption, H2 temperature programmed reduction (H2-TPR), and CO diffuse reflectance infrared Fourier transformed spectroscopy (CO-DRIFTS) show that the Pd was uniformly distributed over the MgAl2O4 support due to strong interaction between Pd and MgAl2O4. The mechanism studies show that the abundant Pd-MgAl2O4 interfaces significantly contributed to the reaction enhancement. The Pd-MgAl2O4 interfaces could greatly enhance the oxidation reaction at a lower temperature with the assistance of oxygen vacancies compared with traditional oxide catalysts, which was confirmed by methanol temperature program surface reaction (MeOH-TPSR) experiments. In-situ DRIFTS is carried out to elucidate the reaction mechanism and establish the structure − activity relationship: the methanol could be effectively absorbed on the MgAl2O4 support with oxygen vacancies to form bidentate formate, then the Pd species assisted the intermediates converting to CO2 product. The Pd/MgAl2O4 catalyst and its enhancement mechanism investigation provided a potential strategy in the VOCs removal catalysis development.
Graphical Abstract








Similar content being viewed by others
References
Billionnet C, Gay E, Kirchner S, Leynaert B, Annesi-Maesano I (2011) Quantitative assessments of indoor air pollution and respiratory health in a population-based sample of French dwellings. Environ Res 111(3):425–434. https://doi.org/10.1016/j.envres.2011.02.008
Hosseini M, Barakat T, Cousin R, Aboukaïs A, Su BL, De Weireld G, Siffert S (2012) Catalytic performance of core–shell and alloy Pd–Au nanoparticles for total oxidation of VOC: the effect of metal deposition. Appl Catal B 111–112:218–224. https://doi.org/10.1016/j.apcatb.2011.10.002
Calzada LA, Collins SE, Han CW, Ortalan V, Zanella R (2017) Synergetic effect of bimetal-lic Au-Ru/TiO2 catalysts for complete oxidation of methanol. Appl Catal B 207:79–92. https://doi.org/10.1016/j.apcatb.2017.01.081
Luo Y, Xiao Y, Cai G, Zheng Y, Wei K (2012) Performance of Ce0.25Zr0.75O2 promoted Pd/Ag/γ-Al2O3 catalysts for low-temperature methanol oxidation. Fuel 93:533–538. https://doi.org/10.1016/j.fuel.2011.10.027
Kim M-Y, Kyriakidou EA, Choi J-S, Toops TJ, Binder AJ, Thomas C, Parks JE, Schwartz V, Chen J, Hensley DK (2016) Enhancing low-temperature activity and durability of Pd-base diesel oxidation catalysts using ZrO2 supports. Appl Catal B 187:181–194. https://doi.org/10.1016/j.apcatb.2016.01.023
Kim C, Hong E, Shin C-H (2019) Improvement of methane combustion activity for Pd/ZrO2 catalyst by simple reduction/reoxidation treatment. Catalysts 9(10):838. https://doi.org/10.3390/catal9100838
Dasireddy VDBC, Likozar B (2019) The role of copper oxidation state in Cu/ZnO/Al2O3 catalysts in CO2 hydrogenation and methanol productivity. Renew Energ 140:452–460. https://doi.org/10.1016/j.renene.2019.03.073
Li Q, Li F-t (2020) Recent advances in surface and interface design of photocatalysts for the degradation of volatile organic compounds. Adv Colloid Interface Sci 284:102275. https://doi.org/10.1016/j.cis.2020.102275
Huang G, Liu L, Chen L, Gao L, Zhu J, Fu H (2022) Unique insights into photocatalytic VOCs oxidation over WO3/carbon dots nanohybrids assisted by water activation and electron transfer at interfaces. J Hazard Mater 423:127134. https://doi.org/10.1016/j.jhazmat.2021.127134
Mahmood A, Shi G, Wang Z, Rao Z, Xiao W, Xie X, Sun J (2021) Carbon quantum dots-TiO2 nanocomposite as an efficient photocatalyst for the photodegradation of aromatic ring-containing mixed VOCs: An experimental and DFT studies of adsorption and electronic structure of the interface. J Hazard Mater 401:123402. https://doi.org/10.1016/j.jhazmat.2020.123402
Wang Q, Li Y, Serrano-Lotina A, Han W, Portela R, Wang R, Banares MA, Yeung KL (2021) Operando investigation of toluene oxidation over 1D Pt@CeO2 derived from Pt cluster-containing MOF. J Am Chem Soc 143(1):196–205. https://doi.org/10.1021/jacs.0c08640
Wang Q, Li Z, Banares MA, Weng LT, Gu Q, Price J, Han W, Yeung KL (2019) A novel approach to high-performance aliovalent-substituted catalysts-2D bimetallic MOF-derived CeCuOx microsheets. Small 15(42):e1903525. https://doi.org/10.1002/smll.201903525
Jabłońska M, Król A, Kukulska-Zając E, Tarach K, Girman V, Chmielarz L, Góra-Marek K (2015) Zeolites Y modified with palladium as effective catalysts for low-temperature methanol incineration. Appl Catal B 166–167:353–365. https://doi.org/10.1016/j.apcatb.2014.11.047
Zhang Z, Chen M, Jiang Z, Shangguan W (2011) Low-temperature selective catalytic reduction of NO with propylene in excess oxygen over the Pt/ZSM-5 catalyst. J Hazard Mater 193:330–334. https://doi.org/10.1016/j.jhazmat.2011.07.038
Kucherov AV, Sinev IM, Ojala S, Keiski R, Kustov LM (2007) Adsorptive-catalytic removal of CH3OH, CH3SH, and CH3SSCH3 from air over the bifunctional system noble metals/HZSM-5. Stud Surf Sci Catal 170:1129–1136. https://doi.org/10.1016/S0167-2991(07)80969-4
Fan L, Wang K, Xu K, Liang Z, Wang H, Zhou SF, Zhan G (2020) Structural isomerism of two Ce-BTC for fabricating Pt/CeO2 nanorods toward low-temperature CO oxidation. Small 16(40):e2003597. https://doi.org/10.1002/smll.202003597
Zhao Q, Yan Z, Chen C, Chen J (2017) Spinels: controlled preparation, oxygen reduction/evolution reaction application, and beyond. Chem Rev 117(15):10121–10211. https://doi.org/10.1021/acs.chemrev.7b00051
Liu T, Xu D, Wu D, Liu G, Hong X (2021) Spinel ZnFe2O4 regulates copper sites for CO2 hydrogenation to methanol. ACS Sustain Chem Eng 9(11):4033–4041. https://doi.org/10.1021/acssuschemeng.0c07682
Dasireddy VDBC, Neja SŠ, Blaž L (2018) Correlation between synthesis pH, structure and Cu/MgO/Al2O3 heterogeneous catalyst activity and selectivity in CO2 hydrogenation to methanol. J CO2 Util 28:189–199. https://doi.org/10.1016/j.jcou.2018.09.002
Dasireddy VDBC, Likozar B (2022) Cu–Mn–O nano-particle/nano-sheet spinel-type materials as catalysts in methanol steam reforming (MSR) and preferential oxidation (PROX) reaction for purified hydrogen production. Renew Energ 182:713–724. https://doi.org/10.1016/j.renene.2021.10.033
Kaczmarczyk J, Zasada F, Janas J, Indyka P, Piskorz W, Kotarba A, Sojka Z (2016) Thermodynamic stability, redox properties, and reactivity of Mn3O4, Fe3O4, and Co3O4 model catalysts for N2O decomposition: Resolving the origins of steady turnover. ACS Catal 6(2):1235–1246. https://doi.org/10.1021/acscatal.5b02642
PalDey S, Gedevanishvili S, Zhang W, Rasouli F (2005) Evaluation of a spinel based pigment system as a CO oxidation catalyst. Appl Catal B 56(3):241–250. https://doi.org/10.1016/j.apcatb.2004.09.013
Evdou A, Zaspalis V, Nalbandian L (2016) Ferrites as redox catalysts for chemical looping processes. Fuel 165:367–378. https://doi.org/10.1016/j.fuel.2015.10.049
Martin A, Luck F, Armbruster U, Patria L, Radnik J, Schneider M (2005) Ammonia removal from effluent streams of wet oxidation under high pressure. Top Catal 33(1–4):155–169. https://doi.org/10.1007/s11244-005-2522-4
Araiza DG, Gómez-Cortés A, Díaz G (2017) Reactivity of methanol over copper supported on well-shaped CeO2: A TPD-DRIFTS study. Catal Sci Technol 7(22):5224–5235. https://doi.org/10.1039/C7CY00984D
Cao T, You R, Zhang X, Chen S, Li D, Zhang Z, Huang W (2018) An in situ DRIFTS mechanistic study of CeO2-catalyzed acetylene semihydrogenation reaction. Phys Chem Chem Phys 20(14):9659–9670. https://doi.org/10.1039/C8CP00668G
Huttunen PK, Labadini D, Hafiz SS, Gokalp S, Wolff EP, Martell SM, Foster M (2021) DRIFTS investigation of methanol oxidation on CeO2 nanoparticles. Appl Surf Sci. https://doi.org/10.1016/j.apsusc.2021.149518
Kattel S, Yan B, Yang Y, Chen JG, Liu P (2016) Optimizing binding energies of key intermediates for CO2 hydrogenation to methanol over oxide-supported copper. J Am Chem Soc 138(38):12440–12450. https://doi.org/10.1021/jacs.6b05791
Bahmanpour AM, Héroguel F, Kılıç M, Baranowski CJ, Schouwink P, Röthlisberger U, Luterbacher JS, Kröcher O (2020) Essential role of oxygen vacancies of Cu-Al and Co-Al spinel oxides in their catalytic activity for the reverse water gas shift reaction. Appl Catal B 266:118669. https://doi.org/10.1016/j.apcatb.2020.118669
Castellanos-Beltran IJ, Perreault L-S, Braidy N (2021) Application of Ni–spinel in the chemical-looping conversion of CO2 to CO via induction-generated oxygen vacancies. J Phys Chem C 125(13):7213–7226. https://doi.org/10.1021/acs.jpcc.1c00928
Wang W, Zhang H-b, Lin G-d, Xiong Z-t (2000) Study of Ag/La0.6Sr0.4MnO3 catalysts for complete oxidation of methanol and ethanol at low concentrations. Appl Catal B 24(3):219–232. https://doi.org/10.1016/S0926-3373(99)00106-X
Lippits MJ, Boer Iwema RRH, Nieuwenhuys BE (2009) A comparative study of oxidation of methanol on γ-Al2O3 supported group IB metal catalysts. Catal Today 145(1):27–33. https://doi.org/10.1016/j.cattod.2008.07.018
Yang Z, Liang S, Sun L, Hu X, Fang W, Lai W, Yi X (2021) Highly active and stable Pd/MgAl2O4@MgO catalyst with electronic metal-support interaction for selective hydrogenation of isoprene. Fuel. https://doi.org/10.1016/j.fuel.2020.119920
Jiang F, Wang S, Liu B, Liu J, Wang L, Xiao Y, Xu Y, Liu X (2020) In-sights into the influence of CeO2 crystal facet on CO2 hydrogenation to methanol over Pd/CeO2 catalysts. ACS Catal 10(19):11493–11509. https://doi.org/10.1021/acscatal.0c03324
Yang J, Peng M, Ren G, Qi H, Zhou X, Xu J, Deng F, Chen Z, Zhang J, Liu K, Pan X, Liu W, Su Y, Li W, Qiao B, Ma D, Zhang T (2020) A hydrothermally stable irreducible oxide-modified Pd/MgAl2O4 catalyst for methane combustion. Angew Chem Int Ed Engl 59(42):18522–18526. https://doi.org/10.1002/anie.202009050
Bruix A, Migani A, Vayssilov GN, Neyman KM, Libuda J, Illas F (2011) Effects of deposited Pt particles on the reducibility of CeO2(111). Phys Chem Chem Phys 13(23):11384–11392. https://doi.org/10.1039/C1CP20950G
Vayssilov GN, Lykhach Y, Migani A, Staudt T, Petrova GP, Tsud N, Skála T, Bruix A, Illas F, Prince KC, Matolı´n Vr, Neyman KM, Libuda J, (2011) Support nanostructure boosts oxygen transfer to catalytically active platinum nanoparticles. Nat Mater 10(4):310–315. https://doi.org/10.1038/nmat2976
Chin Y-H, Buda C, Neurock M, Iglesia E (2013) Consequences of metal–oxide interconversion for C-H bond activation during CH4 reactions on Pd catalysts. J Am Chem Soc 135(41):15425–15442. https://doi.org/10.1021/ja405004m
Wang Q, Tichit D, Meunier F, Guesmi H (2020) Combined DRIFTS and DFT study of CO adsorption and segregation modes in Pt–Sn nanoalloys. J Phys Chem C 124(18):9979–9989. https://doi.org/10.1021/acs.jpcc.0c01296
Ding D, Xu X, Tian P, Liu X, Xu J, Han Y-F (2018) Promotional effects of Sb on Pd-based catalysts for the direct synthesis of hydrogen peroxide at ambient pressure. Chinese J Catal 39(4):673–681. https://doi.org/10.1016/S1872-2067(18)63031-1
Cao Y, Sui Z, Zhu Y, Zhou X, Chen D (2017) Selective hydrogenation of acetylene over Pd-In/Al2O3 catalyst: promotional effect of indium and composition-dependent performance. ACS Catal 7(11):7835–7846. https://doi.org/10.1021/acscatal.7b01745
Liu J, Wang L, Okejiri F, Luo J, Zhao J, Zhang P, Liu M, Yang S, Zhang Z, Song W, Zhu W, Liu J, Zhao Z, Feng G, Xu C, Dai S (2020) Deep understanding of strong metal interface confinement: a journey of Pd/FeOx catalysts. ACS Catal 10(15):8950–8959. https://doi.org/10.1021/acscatal.0c01447
Chen H, Shuang H, Lin W, Li X, Zhang Z, Li J, Fu J (2021) Tuning interfacial electronic properties of palladium oxide on vacancy-abundant carbon nitride for low-temperature dehydrogenation. ACS Catal 11(10):6193–6199. https://doi.org/10.1021/acscatal.1c00712
Sun K, Lu W, Wang M, Xu X (2004) Characterization and catalytic performances of La doped Pd/CeO2 catalysts for methanol decomposition. Appl Catal A 268(1):107–113. https://doi.org/10.1016/j.apcata.2004.03.020
Zhou G-F, Ma J, Bai S, Wang L, Guo Y (2020) CO catalytic oxidation over Pd/CeO2 with different chemical states of Pd. Rare Met 39(7):800–805. https://doi.org/10.1007/s12598-019-01347-7
Chen AL, Yu XJ, Zhou Y, Miao S, Li Y, Kuld S, Sehested J, Liu JY, Aoki T, Hong S, Camellone MF, Fabris S, Ning J, Jin CC, Yang CW, Nefedov A, Woll C, Wang YM, Shen WJ (2019) Structure of the catalytically active copper-ceria interfacial perimeter. Nat Catal 2(4):334–341. https://doi.org/10.1038/s41929-019-0226-6
Cao S, Yang M, Elnabawy AO, Trimpalis A, Li S, Wang C, Goltl F, Chen Z, Liu J, Shan J, Li M, Haas T, Chapman KW, Lee S, Allard LF, Mavrikakis M, Flytzani-Stephanopoulos M (2019) Single-atom gold oxo-clusters prepared in alkaline solutions catalyse the heterogeneous methanol self-coupling reactions. Nat Chem 11(12):1098–1105. https://doi.org/10.1038/s41557-019-0345-3
Dostagir NHMD, Rattanawan R, Gao M, Ota J, Hasegawa J-y, Asakura K, Fukouka A, Shrotri A (2021) Co single atoms in ZrO2 with inherent oxygen vacancies for selective hydrogenation of CO2 to CO. ACS Catal 11(15):9450–9461. https://doi.org/10.1021/acscatal.1c02041
Yue Y, Li Y, Wang T, Wang S, Han L, Du C (2022) Enhancement of methanol oxidation performance over Pd/CeO2 derived from MOF and mechanism investigation via in situ studies. Catal Lett. https://doi.org/10.1007/s10562-021-03901-4
Acknowledgements
This work was supported by the National Natural Science Foundation of China [Grant Number 42107054].
Funding
National Natural Science Foundation of China,42107054
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, W., Zhang, H., Zhang, L. et al. Methanol Oxidation Catalytic Performance Enhancement via Constructing Pd-MgAl2O4 Interface and its Reaction Mechanism Investigation. Catal Lett 153, 1786–1796 (2023). https://doi.org/10.1007/s10562-022-04107-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04107-y