Abstract
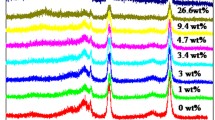
Sn-β samples with nSi/nSn from 1600 to 100 were hydrothermally synthesized, characterized and tested for transforming glucose to methyl lactate (MLA). Both the amount of framework Sn and extraframework Sn increased with raising Sn content in the synthesis gel; moreover the silanol defects also increased. For converting glucose to MLA, the TOF value of MLA production reduced as the Sn content rose due to the increased silanol defects and extraframework Sn. The maximum MLA productivity (~ 104 g kgcatalyst‒1 h‒1) was achieved at nSi/nSn of 400–200 when the glucose concentration was 9.3 wt%. During four sequence runs, the MLA yield increased over Sn-β with nSi/nSn of 100–400, while it reduced over Sn-β with nSi/nSn of 800–1600. The different recycling behavior of Sn-β with different nSi/nSn was revealed.
Graphical Abstract












Similar content being viewed by others
References
Fan Y, Zhou C, Zhu X (2009) Selective catalysis of lactic acid to produce commodity chemicals. Catal Rev 51:293–324
Dusselier M, Wouwe PV, Dewaele A, Makshina E, Sels BF (2013) Lactic acid as a platform chemical in the biobased economy: the role of chemocatalysis. Energ Environ Sci 6:1415–1442
Gallezot P (2012) Conversion of biomass to selected chemical products. Chem Soc Rev 41:1538–1558
Datta R, Henry M (2006) Lactic acid: recent advances in products, processes and technologies-a review. J Chem Technol Biotechnol 81:1119–1129
Nampoothiri KM, Nair NR, John RP (2010) An overview of the recent developments in polylactide (PLA) research. Bioresour Technol 101:8493–8501
Bozell JJ, Petersen GR (2010) Technology development for the production of biobased products from biorefinery carbohydrates-the US Department of Energy’s “Top 10” revisited. Green Chem 12:539–554
Sandra R, Javier L, Adriana P (2017) Impregnation of kraft paper support with polylactic acid multilayers. Adv Mat Lett 8:741–751
Ilyas RA, Sapuan SM, Harussani MM, Hakimi MYAY, Haziq MZM, Atikah MSN, Asyraf MRM, Ishak MR, Razman MR, Nurazzi NM, Norrrahim MNF, Abral H, Asrofi M (2021) Polylactic acid (PLA) biocomposite: Processing, additive manufacturing and advanced applications. Polymers 13:1326–1359
Corma A, Iborra S, Velty A (2007) Chemical routes for the transformation of biomass into chemicals. Chem Rev 107:2411–2502
Chheda JN, Huber GW, Dumesic JA (2007) Liquid-phase catalytic processing of biomass-derived oxygenated hydrocarbons to fuels and chemicals. Angew Chem Int Ed 46:7164–7183
Li S, Deng W, Li Y, Zhang Q, Wang Y (2019) Catalytic conversion of cellulose-based biomass and glycerol to lactic acid. J Energ Chem 32:138–151
Zhou L, Wu L, Li H, Yang X, Su Y, Lu T, Xu J (2014) A facile and efficient method to improve the selectivity of methyl lactate in the chemocatalytic conversion of glucose catalyzed by homogeneous Lewis acid. J Mol Catal A: Chem 388–389:74–80
Bicker M, Endres S, Ott L, Vogel H (2005) Catalytical conversion of carbohydrates in subcritical water: a new chemical process for lactic acid production. J Mol Catal A: Chem 239:151–157
Holm MS, Saravanamurugan S, Taarning E (2010) Conversion of sugars to lactic acid derivatives using heterogeneous zeotype catalysts. Science 328:602–605
Rasrendra CB, Makertihartha IGBN, Adisasmito S, Heeres HJ (2010) Green chemicals from d-glucose: systematic studies on catalytic effects of inorganic salts on the chemo-selectivity and yield in aqueous solutions. Top Catal 53:1241–1247
Wang F, Liu C, Dong W (2013) Highly efficient production of lactic acid from cellulose using lanthanide triflate catalysts. Green Chem 15:2091–2095
Liu F, Huang K, Zheng A, Xiao F, Dai S (2017) Hydrophobic solid acids and their catalytic applications in green and sustainable chemistry. ACS Catal 8:372–391
Lei X, Wang F, Liu C, Yang R, Dong W (2014) One-pot catalytic conversion of carbohydrate biomass to lactic acid using an ErCl3 catalyst. Appl Catal A: Gen 482:78–83
Wang J, Yao G, Jin F (2017) One-pot catalytic conversion of carbohydrates into alkyl lactates with Lewis acids in alcohols. Mol Catal 435:82–90
Tang Z, Deng W, Wang Y, Zhu E, Wan X, Zhang Q, Wang Y (2014) Transformation of cellulose and its derived carbohydrates into formic and lactic acids catalyzed by vanadyl cations. ChemSusChem 7:1557–1567
Roman-Leshkov Y, Moliner M, Labinger JA, Davis ME (2010) Mechanism of glucose isomerization using a solid Lewis acid catalyst in water. Angew Chem Int Ed 49:8954–8957
Tolborg S, Sadaba I, Osmundsen CM, Fristrup P, Holm MS, Taarning E (2015) Tin-containing silicates: alkali salts improve methyl lactate yield from sugars. ChemSusChem 8:613–617
Moliner M, Roman-Leshkov Y, Davis ME (2010) Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. P Natl Acad Sci USA 107:6164–6168
Lew CM, Rajabbeigi N, Tsapatsis M (2012) Tin-containing zeolite for the isomerization of cellulosic sugars. Microporous Mesoporous Mater 153:55–58
Tolborg S, Katerinopoulou A, Falcone DD, Sadaba I, Osmundsen CM, Davis RJ, Taarning E, Fristrup P, Holm MS (2014) Incorporation of tin affects crystallization, morphology, and crystal composition of Sn-Beta. J Mater Chem A 2:20252–20262
Corma A, Nemeth LT, Renz M, Valencia S (2001) Sn-zeolite beta as a heterogeneous chemoselective catalyst for Baeyer-Villiger oxidations. Nature 412:423–425
Tang B, Dai W, Wu G, Guan N, Li L, Hunger M (2014) Improved postsynthesis strategy to Sn-Beta zeolites as Lewis acid catalysts for the ring-opening hydration of epoxides. ACS Catal 4:2801–2810
Li P, Liu G, Wu H, Liu Y, Jiang J, Wu P (2011) Postsynthesis and selective oxidation properties of nanosized Sn-Beta zeolite. J Phys Chem C 115:3663–3670
Dijkmans J, Gabriëls D, Dusselier M, de Clippel F, Vanelderen P, Houthoofd K, Malfliet A, Pontikes Y, Sels BF (2013) Productive sugar isomerization with highly active Sn in dealuminated β zeolites. Green Chem 15:2777–2785
van der Graaff WNP, Li G, Brahim BM, Pidko EA, Hensen EJM (2015) Synthesis of Sn-Beta with exclusive and high framework Sn content. ChemCatChem 7:1152–1160
Bermejo-Deval R, Gounder R, Davis ME (2012) Framework and extraframework tin sites in zeolite beta react glucose differently. ACS Catal 2:2705–2713
Hammond C, Conrad S, Hermans I (2012) Simple and scalable preparation of highly active Lewis acidic Sn-beta. Angew Chem Int Ed 51:11736–11739
Kang Z, Zhang X, Liu H, Qiu J, Yeung K (2013) A rapid synthesis route for Sn-Beta zeolites by steam-assisted conversion and their catalytic performance in Baeyer-Villiger oxidation. Chem Eng J 218:425–432
Wolf P, Valla M, Núñez-Zarur F, Comas-Vives A, Rossini AJ, Firth C, Kallas H, Lesage A, Emsley L, Copéret C, Hermans I (2016) Correlating synthetic methods, morphology, atomic-level structure and catalytic activity of Sn-β catalysts. ACS Catal 6:4047–4063
Wolf P, Liao W, Ong T, Valla M, Harris JW, Gounder R, van der Graaff WNP, Pidko EA, Hensen EJM, Ferrini P, Dijkmans J, Sels B, Hermans I, Copéret C (2016) Identifying Sn site heterogeneities prevalent among Sn-Beta zeolites. Helv Chim Acta 99:916–927
Vega-Vila JC, Harris JW, Gounder R (2016) Controlled insertion of tin atoms into zeolite framework vacancies and consequences for glucose isomerization catalysis. J Catal 344:108–120
Conrad S, Wolf P, Müller P, Orsted H, Hermans I (2017) Influence of hydrophilicity on the Snβ-catalyzed Baeyer-Villiger oxidation of cyclohexanone with aqueous hydrogen peroxide. ChemCatChem 9:175–182
Chang C, Cho HJ, Wang Z, Wang X, Fan W (2015) Fluoride-free synthesis of a Sn-BEA catalyst by dry gel conversion. Green Chem 17:2943–2951
Xu H, Wang X, Ji P, Wu H, Guan Y, Wu P (2018) Hydrothermal synthesis of Sn-Beta zeolites in F−-free medium. Inorg Chem Front 5:2763–2771
Hwang SJ, Gounder R, Bhawe Y, Orazov M, Bermejo-Deval R, Davis ME (2015) Solid state NMR characterization of Sn-Beta zeolites that catalyze glucose isomerization and epimerization. Top Catal 58:435–440
Yang X, Liu Y, Li X, Ren J, Zhou L, Lu T, Su Y (2018) Synthesis of Sn-containing nanosized Beta zeolite as efficient catalyst for transformation of glucose to methyl lactate. ACS Sustain Chem Eng 6:8256–8265
Yang X, Wang L, Lu T, Gao B, Su Y, Zhou L (2020) Seed-assisted hydrothermal synthesis of Sn-Beta for conversion of glucose to methyl lactate: effects of the H2O amount in the gel and crystallization time. Catal Sci Technol 10:8437–8444
Osmundsen CM, Holm MS, Dahl S, Taarning E (2012) Tin-containing silicates: structure-activity relations. P Roy Soc A-Math Phy 468:2000–2016
Larlus O, Valtchev VP (2005) Control of the morphology of all-silica BEA-type zeolite synthesized in basic media. Chem Mater 17:881–886
Yakimov AV, Kolyagin YG, Tolborg S, Vennestrøm PNR, Ivanova II (2016) Accelerated synthesis of Sn-BEA in fluoride media: effect of H2O content in the gel. New J Chem 40:4367–4374
Paris C, Moliner M, Corma A (2013) Metal-containing zeolites as efficient catalysts for the transformation of highly valuable chiral biomass-derived products. Green Chem 15:2101–2109
Harris JW, Cordon MJ, Iorio JRD, Vega-Vila JC, Ribeiro FH, Gounder R (2016) Titration and quantification of open and closed Lewis acid sites in Sn-Beta zeolites that catalyze glucose isomerization. J Catal 335:141–154
Otomo R, Kosugi R, Kamiya Y, Tatsumi T, Yokoi T (2016) Modification of Sn-Beta zeolite: characterization of acidic/basic properties and catalytic performance in Baeyer-Villiger oxidation. Catal Sci Technol 6:2787–2795
Yang X, Bian J, Huang J, Xin W, Lu T, Chen C, Su Y, Zhou L, Wang F, Xu J (2017) Fluoride-free and low concentration template synthesis of hierarchical Sn-Beta zeolites: efficient catalysts for conversion of glucose to alkyl lactate. Green Chem 19:692–701
Yang X, Lv B, Lu T, Su Y, Zhou L (2020) Promotion effect of Mg on post-synthesized Sn-Beta zeolite for the conversion of glucose to methyl lactate. Catal Sci Technol 10:700–709
Hammond C, Padovan D, Al-Nayili A, Wells PP, Gibson EK, Dimitratos N (2015) Identification of cctive and spectator Sn sites in Sn-β following solid-state stannation, and consequences for Lewis acid catalysis. ChemCatChem 7:3322–3331
van der Graaff WNP, Tempelman CHL, Hendriks FC, Ruiz-Martinez J, Bals S, Weckhuysen BM, Pidko EA, Hensen EJM (2018) Deactivation of Sn-Beta during carbohydrate conversion. Appl Catal A: Gen 564:113–122
Vega-Vila JC, Gounder R (2020) Quantification of intraporous hydrophilic binding sites in Lewis acid zeolites and consequences for sugar isomerization catalysis. ACS Catal 10:12197–12211
Acknowledgements
We appreciate the financial support from the National Natural Science Foundation of China (Grant No. 21871236). The program of Young Key Teacher of Universities in Henan Province (Grant No. 2019GGJS015) is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that there are no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, X., Wang, Y., Su, Y. et al. Influence of Sn Content in Sn-β on Selective Production of Methyl Lactate from Glucose. Catal Lett 153, 1773–1785 (2023). https://doi.org/10.1007/s10562-022-04101-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-022-04101-4