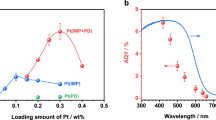
Abstract
Photocatalytic reduction of CO2 with water to valuable chemicals has been getting attention as an artificial photosynthetic system using CO2 as a resource. A barium tetratitanate, BaTi4O9, loaded with Ag nanoparticles (Ag/BaTi4O9) successfully promoted the photocatalytic CO2 reduction to yield CO with very high selectivity (> 99%) in an aqueous solution.
Graphic Abstract






Similar content being viewed by others
References
Habisreutinger SN, Schmidt-Mende L, Stolarczyk JK (2013) Photocatalytic reduction of CO2 on TiO2 and other semiconductors. Angew Chem Int Ed 52:7372–7408. https://doi.org/10.1002/anie.201207199
Lingampalli SR, Ayyub MM, Rao CNR (2017) Recent progress in the photocatalytic reduction of carbon dioxide. ACS Omega 2:2740–2748. https://doi.org/10.1021/acsomega.7b00721
Iizuka K, Wato T, Miseki Y, Saito K, Kudo A (2011) Photocatalytic reduction of carbon dioxide over Ag cocatalyst-loaded ALa4Ti4O15 (A = Ca, Sr, and Ba) using water as a reducing reagent. J Am Chem Soc 133:20863–20868. https://doi.org/10.1021/ja207586e
Takayama T, Iwase A, Kudo A (2015) Photocatalytic water splitting and CO2 reduction over KCaSrTa5O15 nanorod prepared by a polymerized complex method. Bull Chem Soc Jpn 88:538–543. https://doi.org/10.1246/bcsj.20140350
Nakanishi H, Iizuka K, Takayama T, Iwase A, Kudo A (2017) Highly active NaTaO3-based photocatalysts for CO2 reduction to form CO using water as the electron donor. ChemSusChem 10:112–118. https://doi.org/10.1002/cssc.201601360
Takayama T, Nakanishi H, Matsui M, Iwase A, Kudo A (2018) Photocatalytic CO2 reduction using water as an electron donor over Ag-loaded metal oxide photocatalysts consisting of several polyhedra of Ti4+, Zr4+, and Ta5+. J Photochem Photobiol A Chem 358:416–421. https://doi.org/10.1016/j.jphotochem.2017.10.002
Wang Z, Teramura K, Hosokawa S, Tanaka T (2015) Photocatalytic conversion of CO2 in water over Ag-modified La2Ti2O7. Appl Catal B 163:241–247. https://doi.org/10.1016/j.apcatb.2014.07.052
Wang Z, Teramura K, Hosokawa S, Tanaka T (2015) Highly efficient photocatalytic conversion of CO2 into solid CO using H2O as a reductant over Ag-modified ZnGa2O4. J Mater Chem A 3:11313–11319. https://doi.org/10.1039/c5ta01697e
Teramura K, Wang Z, Hosokawa S, Sakata Y, Tanaka T (2014) A doping technique that suppresses undesirable H2 evolution derived from overall water splitting in the highly selective photocatalytic conversion of CO2 in and by water. Chemistry 20:9906–9909. https://doi.org/10.1002/chem.201402242
Wang Z, Teramura K, Huang Z, Hosokawa S, Sakata Y, Tanaka T (2016) Tuning the selectivity toward CO evolution in the photocatalytic conversion of CO2 with H2O through the modification of Ag-loaded Ga2O3 with a ZnGa2O4 layer. Catal Sci Technol 6:1025–1032. https://doi.org/10.1039/c5cy01280e
Teramura K, Tatsumi H, Wang Z, Hosokawa S, Tanaka T (2015) Photocatalytic conversion of CO2 by H2O over Ag-loaded SrO-modified Ta2O5. Bull Chem Soc Jpn 88:431–437. https://doi.org/10.1246/bcsj.20140385
Iguchi S, Teramura K, Hosokawa S, Tanaka T (2016) A ZnTa2O6 photocatalyst synthesized via solid state reaction for conversion of CO2 into CO in water. Catal Sci Technol 6:4978–4985. https://doi.org/10.1039/c6cy00271d
Huang Z, Teramura K, Hosokawa S, Tanaka T (2016) Fabrication of well-shaped Sr2KTa5O15 nanorods with a tetragonal tungsten bronze structure by a flux method for artificial photosynthesis. Appl Catal B 199:272–281. https://doi.org/10.1016/j.apcatb.2016.06.039
Pang R, Teramura K, Asakura H, Hosokawa S, Tanaka T (2017) Highly selective photocatalytic conversion of CO2 by water over Ag-loaded SrNb2O6 nanorods. Appl Catal B 218:770–778. https://doi.org/10.1016/j.apcatb.2017.06.052
Iguchi S, Hasegawa Y, Teramura K, Kidera S, Kikkawa S, Hosokawa S, Asakura H, Tanaka T (2017) Drastic improvement in the photocatalytic activity of Ga2O3 modified with Mg–Al layered double hydroxide for the conversion of CO2 in water. Sustain Energy Fuels 1:1740–1747. https://doi.org/10.1039/C7SE00204A
Huang Z, Teramura K, Asakura H, Hosokawa S, Tanaka T (2017) CO2 capture, storage, and conversion using a praseodymium-modified Ga2O3 photocatalyst. J Mater Chem A 5:19351–19357. https://doi.org/10.1039/c7ta04918h
Huang Z, Teramura K, Asakura H, Hosokawa S, Tanaka T (2018) Flux method fabrication of potassium rare-earth tantalates for CO2 photoreduction using H2O as an electron donor. Catal Today 300:173–182. https://doi.org/10.1016/j.cattod.2017.03.043
Tatsumi H, Teramura K, Huang Z, Wang Z, Asakura H, Hosokawa S, Tanaka T (2017) Enhancement of CO evolution by modification of Ga2O3 with rare-earth elements for the photocatalytic conversion of CO2 by H2O. Langmuir 33:13929–13935. https://doi.org/10.1021/acs.langmuir.7b03191
Rui P, Teramura K, Tatsumi H, Asakura H, Hosokawa S, Tanaka T (2018) Modification of Ga2O3 by an Ag–Cr core–shell cocatalyst enhances photocatalytic CO evolution for the conversion of CO2 by H2O. Chem Commun 54:1053–1056. https://doi.org/10.1039/c7cc07800e
Wang S, Teramura K, Hisatomi T, Domen K, Asakura H, Hosokawa S, Tanaka T (2020) Optimized synthesis of Ag-modified Al-doped SrTiO3 photocatalyst for the conversion of CO2 using H2O as an electron donor. ChemistrySelect 5:8779–8786. https://doi.org/10.1002/slct.202001693
Wang S, Teramura K, Hisatomi T, Domen K, Asakura H, Hosokawa S, Tanaka T (2020) Effective driving of Ag-loaded and Al-doped SrTiO3 under irradiation at λ > 300 nm for the photocatalytic conversion of CO2 by H2O. ACS Appl Energy Mater 3:1468–1475. https://doi.org/10.1021/acsaem.9b01927
Yoshida H, Zhang L, Sato M, Morikawa T, Kajino T, Sekito T, Matsumoto S, Hirata H (2015) Calcium titanate photocatalyst prepared by a flux method for reduction of carbon dioxide with water. Catal Today 251:132–139. https://doi.org/10.1016/j.cattod.2014.10.039
Anzai A, Fukuo N, Yamamoto A, Yoshida H (2017) Highly selective photocatalytic reduction of carbon dioxide with water over silver-loaded calcium titanate. Catal Commun 100:134–138. https://doi.org/10.1016/j.catcom.2017.06.046
Yoshida H, Sato M, Fukuo N, Zhang L, Yoshida T, Yamamoto Y, Morikawa T, Kajino T, Sakano M, Sekito T, Matsumoto S, Hirata H (2018) Sodium hexatitanate photocatalysts prepared by a flux method for reduction of carbon dioxide with water. Catal Today 303:296–304. https://doi.org/10.1016/j.cattod.2017.09.029
Zhu X, Anzai A, Yamamoto A, Yoshida H (2019) Silver-loaded sodium titanate photocatalysts for selective reduction of carbon dioxide to carbon monoxide with water. Appl Catal B 243:47–56. https://doi.org/10.1016/j.apcatb.2018.10.021
Zhu X, Yamamoto A, Imai S, Tanaka A, Kominami H, Yoshida H (2019) A silver-manganese dual co-catalyst for selective reduction of carbon dioxide into carbon monoxide over a potassium hexatitanate photocatalyst with water. Chem Commun 55:13514–13517. https://doi.org/10.1039/c9cc06038c
Zhu X, Yamamoto A, Imai S, Tanaka A, Kominami H, Yoshida H (2020) Facet-selective deposition of a silver–manganese dual cocatalyst on potassium hexatitanate photocatalyst for highly selective reduction of carbon dioxide by water. Appl Catal B 274:119085. https://doi.org/10.1016/j.apcatb.2020.119085
Yamashita Y, Yoshida K, Kakihana M, Uchida S, Sato T (1999) Polymerizable complex synthesis of RuO2/BaTi4O9 photocatalysts at reduced temperatures: factors affecting the photocatalytic activity for decomposition of water. Chem Mater 11:61–66. https://doi.org/10.1021/cm9804012
Inoue Y, Asai Y, Sato K (1994) Photocatalysts with tunnel structures for decomposition of water. Part 1.—BaTi4O9, a pentagonal prism tunnel structure, and its combination with various promoters. J Chem Soc Faraday Trans 90:797–802
Kohno M, Kaneko T, Ogura S, Sato K, Inoue Y (1998) Dispersion of ruthenium oxide on barium titanates (Ba6Ti17O40, Ba4Ti13O30, BaTi4O9 and Ba2Ti9O20) and photocatalytic activity for water decomposition. J Chem Soc Faraday Trans 94:89–94. https://doi.org/10.1039/a704947a
Kohno M, Ogura S, Inoue Y (1996) Preparation of BaTi4O9 by a sol-gel method and its photocatalytic activity for water decomposition. J Mater Chem 6:1921–1924. https://doi.org/10.1039/JM9960601921
Ogura S, Sato K, Inoue Y (2000) Effects of RuO2 dispersion on photocatalytic activity for water decomposition of BaTi4O9 with a pentagonal prism tunnel and K2Ti4O9 with a zigzag layer structure. Phys Chem Chem Phys 2:2449–2454. https://doi.org/10.1039/b000187m
Arima M, Kakihana M, Sato T, Yoshida K, Yamashita Y, Yashima M, Yoshimura M (1996) Highly active BaTi4O9/RuO2 photocatalyst by polymerized complex method. Appl Phys Lett 69:2053–2055. https://doi.org/10.1063/1.116877
Hiramachi Y, Fujimori H, Yamakata A, Sakata Y (2019) Achievement of high photocatalytic performance to BaTi4O9 toward overall H2O splitting. ChemCatChem 11:6213–6217. https://doi.org/10.1002/cctc.201901564
Zhang X, Tang S, Li R, Du Y (2013) Synthesis and photocatalytic property of BaTi4O9/RuO2 nanocomposites. Mater Res Bull 48:609–612. https://doi.org/10.1016/j.materresbull.2012.11.039
Kohno M, Ogura S, Sato K, Inoue Y (1996) Effect of tunnel structures of BaTi4O9 and Na2Ti6O13 on photocatalytic activity and photoexcited charge separation. Stud Surf Sci Catal 101:143–152. https://doi.org/10.1016/S0167-2991(96)80224-2
Sato J, Kobayashi H, Inoue Y (2003) Photocatalytic activity for water decomposition of indates with octahedrally coordinated d10 configuration. II. Roles of geometric and electronic structures. J Phys Chem B 107:7970–7975. https://doi.org/10.1021/jp030021q
Lou Z, Wang P, Huang B, Dai Y, Qin X, Zhang X, Wang Z, Liu Y (2017) Enhancing charge separation in photocatalysts with internal polar electric fields. ChemPhotoChem 1:136–147. https://doi.org/10.1002/cptc.201600057
Guo Y, Shi W, Zhu Y (2019) Internal electric field engineering for steering photogenerated charge separation and enhancing photoactivity. EcoMat 1:e12007. https://doi.org/10.1002/eom2.12007
Hu Y, Pan Y, Wang Z, Lin T, Gao Y, Luo B, Hu H, Fan F, Liu G, Wang L (2020) Lattice distortion induced internal electric field in TiO2 photoelectrode for efficient charge separation and transfer. Nat Commun 11:1–10. https://doi.org/10.1038/s41467-020-15993-4
Tauc J, Grigorovici R, Vancu A (1966) Optical properties and electronic structure of amorphous germanium. Phys Status Solidi 15:627–637. https://doi.org/10.1002/pssb.19660150224
Nomura M, Koike Y, Sato M, Koyama A, Inada Y, Asakura K (2007) A new XAFS beamline NW10A at the photon factory. AIP Conf Proc 882:896–898. https://doi.org/10.1063/1.2644697
Teramura K, Hori K, Terao Y, Huang Z, Iguchi S, Wang Z, Asakura H, Hosokawa S, Tanaka T (2017) Which is an intermediate species for photocatalytic conversion of CO2 by H2O as the electron donor: CO2 molecule, carbonic acid, bicarbonate, or carbonate ions? J Phys Chem C 121:8711–8721. https://doi.org/10.1021/acs.jpcc.6b12809
Templeton DH, Dauben CH (1960) Polarized octahedra in barium tetratitanate. J Chem Phys 32:1515–1518. https://doi.org/10.1063/1.1730951
Scaife DE (1980) Oxide semiconductors in photoelectrochemical conversion of solar energy. Sol Energy 25:41–54. https://doi.org/10.1016/0038-092X(80)90405-3
Liou YC, Wu CT, Tseng KH, Chung TC (2005) Synthesis of BaTi4O9 ceramics by reaction-sintering process. Mater Res Bull 40:1483–1489. https://doi.org/10.1016/j.materresbull.2005.04.028
Lowell S, Shields JE (2013) Powder surface area and porosity, 3rd edn. Springer, Berlin
Soltani T, Zhu X, Yamamomo A, Singh SP, Fudo E, Tanaka A, Kominami H, Yoshida H (2021) Effect of transition metal oxide cocatalyst on the photocatalytic activity of Ag loaded CaTiO3 for CO2 reduction with water and water splitting. Appl Catal B 286:119899. https://doi.org/10.1016/j.apcatb.2021.119899
Cui Y, Sun H, Shen G, Jing P, Pu Y (2020) Effect of dual-cocatalyst surface modification on photodegradation activity, pathway, and mechanisms with highly efficient Ag/BaTiO3/MnOx. Langmuir 36:498–509. https://doi.org/10.1021/acs.langmuir.9b02714
Ishii T, Anzai A, Yamamoto A, Yoshida H (2020) Calcium zirconate photocatalyst and silver cocatalyst for reduction of carbon dioxide with water. Appl Catal B 277:119192. https://doi.org/10.1016/j.apcatb.2020.119192
Acknowledgements
The XAFS experiment was performed under the approval of the Photon Factory Program Advisory Committee (Proposal No. 2020G667). This work was financially supported by the joint research program of the Artificial Photosynthesis, Osaka City University, ISHIZUE 2020 of Kyoto University Research Development Program, the Masuya Memorial Basic Research Foundation, and the Program for Element Strategy Initiative for Catalysts & Batteries (ESICB, JPMXP0112101003) commissioned by the MEXT of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Anzai, A., Yamamoto, A. & Yoshida, H. BaTi4O9 Photocatalysts with Variously Loaded Ag Cocatalyst for Highly Selective Photocatalytic CO2 Reduction with Water. Catal Lett 152, 2498–2506 (2022). https://doi.org/10.1007/s10562-021-03831-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03831-1