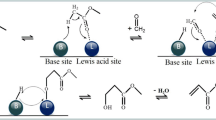
Abstract
A series of ammonium salts of molybdovanadophosphoric acid with different metal substitution and x values (abbreviated as (NH4)1.85Mx, M=Cs, Cu and Fe) were synthesized to catalyze the partial oxidation of isobutane to methacrylic acid. By XRD, TG/DTG, XRF, FT-IR, Raman spectroscopy, H2-TPR, NH3-TPD, it can be found that, compared to the un-doped (NH4)1.85H2.15PMo11VO40 catalyst, the specific surface area, amount of acid sites, and immigrating amount of V atom in Keggin unit into the secondary structure were strongly dependent on the substituted metal ions and their content. The optimum activity was obtained over (NH4)1.85Cs0.5 catalyst, which could provide a larger surface area of 37.72 m2 g−1, a higher amount of acid sites (82.47 μmol g−1), and more VO2+ species and V2O5 clusters from the immigration of V atom in Keggin structure. Furthermore, Cu-substituted catalyst accelerated the catalytic cycle of isobutane oxidation due to the higher electron-delivering efficiency, and the MAA desorption became faster and the catalysts exhibited the excellent selectivity to MAA.
Graphic Abstract








Similar content being viewed by others
References
Ding XL, Wu XN, Zhao YX, He SG (2012) Acc Chem Res 3:382
Vedrine JC (2016) J Energy Chem 25:936
Grant JT, Venegas JM, Mcdermott WP, Hermans I (2017) Chem Rev 118:2769
Pierre CS, Xiu LP, Xin HB (2017) Chem Rev 117:8497
Grasselli RK (2014) Catal Today 238:10
Li B, Yan R, Lei W, Diao YY, Li ZX, Zhang SJ (2013) Catal Lett 143:829
Mahboub MJD, Dubois JL, Cavani F, Rostamizadeh M, Patience GS (2018) Chem Soc Rev 47:7703
Chansai S, Kato Y, Ninomiya W, Hardacre C (2021) Faraday Discuss 229:443
Rajagopalan P, Kuehnle M, Polyakov M, Muller K, Arlt W, Kruse D, Bruckner A, Bentrup U (2014) Catal Commun 48:19
Kendel S, Brown T (2011) Catal Lett 142:1767
Liu SZ, Chen L, Wang GW, Liu JW, Gao YA, Shan HH (2016) J Energy Chem 25:85
Jing FL, Katryniok B, Dumeignil F, Bordes-Richard E, Paul S (2014) Catal Sci Technol 4:2938
He JF, Liu YC, Chu WL, Yang WS (2018) Appl Catal A Gen 556:104
Langpape M, Millet JM, Ozkan US, Delichere P (1999) J Catal 182:148
Min JS, Mizuno N (2001) Catal Today 71:89
Mizuno N, Tateishi M, Iwamoto M (1994) J Chem Soc Chem Commun 1411
Zheng Y, Zhang H, Wang L, Zhang SJ, Wang SJ (2016) Front Chem Sci Eng 10:139
Li XK, Zhao J, Ji WJ, Zhang ZB, Chen Y, Au CT, Han S, Hibst H (2006) J Catal 237:58
Sultan M, Paul S, Fournier M, Vanhove D (2004) Appl Catal A Gen 259:141
Cai X, Ma Y, Zhou Q, Zhang ZT, Chu WL, Yang WS (2021) React Kinet Mech Catal 133:293
Zhou LL, Zhang SS, Li ZJ, Scott J, Zhang ZK, Liu RJ, Yun J (2019) RSC Adv 9:34065
Langpape M, Millet J, Ozkan US, Boudeulle M (1999) J Catal 181:80
Marosi L, AreáN C (2003) J Catal 213:235
Ballarini N, Candiracci F, Cavani F, Degrand H, Dubois JL, Lucarelli G, Margotti M, Patinet A, Pigamo A, Trifiro F (2007) Appl Catal A Gen 325:263
Zhou LL, Lwang L, Zhang SJ, Yan RY, Diao YY (2015) J Catal 329:431
Brückner A, Scholz G, Heidemann D, Schneider M, Herein D, Bentrup U, Kant M (2007) J Catal 245:369
Dimitratos N, Védrine J (2003) Appl Catal A Gen 256:251
Casarini D, Centi G, Jiru P, Lena V, Tvaruzkova Z (1993) J Catal 143:325
Cao YL, Wang L, Zhou LL, Zhang GJ, Xu BH, Zhang SJ (2017) Ind Eng Chem Res 56:653
Jing F, Katryniok B, Dumeignil F, Borders-Richard E, Paul S (2014) J Catal 309:121
Loridant S, Huynh Q, Millet J (2010) Catal Today 155:214
Hardcastle FD, Wachs IE (1991) J Phys Chem 95:5031
Pöppl A, Manikandan P, Köhler K, Maas P, Strauch P, Bottcher R, Doldfarb D (2001) J Am Chem Soc 123:4577
Damyanova S, Cubeiro ML, Fierro JLG (1999) J Mol Catal A Chem 142:85
Zhang H, Yan RY, Yang L, Diao YY, Wang L, Zhang SJ (2013) Ind Eng Chem Res 52:4484
Putluru S, Mossin S, Riisager AF, Fehrmann R (2011) Catal Today 176:292
Liu H, Imoto H, Shido T, Iwasawa Y (2001) J Catal 200:69
Zhou LL, Wang L, Cao YL, Diao YY, Yan RY, Zhang SJ (2017) Mol Catal 438:47
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21673227), Scientific Research Project of Mudanjiang Normal University (No.1352DZ001); Innovation and Entrepreneurship Project of Mudanjiang Normal University (No.201910233025); Science and Technology Innovation Project of Mudanjiang Normal University (No.kjcx2020-024mdjnu).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cai, X., Ma, Y., Chu, W. et al. Selective Oxidation of Isobutane to Methacrylic Acid by Metal-Substituted Ammonium Salts of Molybdovanadophosphoric Acid. Catal Lett 152, 2412–2420 (2022). https://doi.org/10.1007/s10562-021-03821-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03821-3