Abstract
The statistical selectivity models were developed for four different Fischer–Tropsch synthesis product range, including methane (CH4), light olefins (C2=C4), light paraffins (C2–C4), and long-chain hydrocarbons (C5+), based on the experimental data obtained over thirteen γ-Al2O3 supported cobalt-based catalysts with different cobalt particle and pore sizes. The input variables consist of cobalt metal particle size and catalyst pore size. The cubic and quadratic polynomial equations were fitted to the experimental data, however, the mathematical models were subjected to model reduction for the enhancement of model adequacy, which was investigated through ANOVA. The multi-objective optimization revealed that the maximum C5+ selectivity (84.150%) could be achieved at the cobalt particle size and pore sizes of 14.764 and 23.129 nm, respectively, while keeping the selectivity to other hydrocarbon products minimum.
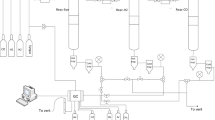
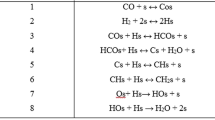
Graphic Abstract





Similar content being viewed by others
References
Ail SS, Dasappa S (2016) Biomass to liquid transportation fuel via Fischer Tropsch synthesis: technology review and current scenario. Renew Sustain Energy Rev 58:267–286. https://doi.org/10.1016/J.RSER.2015.12.143
Klerk A, de Li Y, Zennaro R (2013) Fischer-Tropsch technology. In: Maitlis PM, de Klerk A (eds) Greener Fischer-Tropsch processes for fuels and feedstocks, 1st edn. Wiley, New York, pp 53–79
Dry ME (2004) Chemical concepts used for engineering purposes. In: Steynberg A, Dry M (eds) Studies in surface sciences and catalysis. Elsevier, New York, pp 196–257
de Klerk A (2011) Fischer-Tropsch synthesis. In: de Klerk A (ed) Fischer-Tropsch refining, 1st edn. Wiley, New York, pp 73–103
Khodakov AY, Chu W, Fongarland P (2007) REVIEW Novel cobalt Fischer-Tropsch catalysts for synthesis of long-chain hydrocarbons and clean fuels. Chem Rev 107(5):1692–1744. https://doi.org/10.1021/cr050972v
Mahmoudi H, Mahmoudi M, Doustdar O, Jahangiri H, Tsolakis A, Gu S, LechWyszynski M (2017) A review of Fischer Tropsch synthesis process, mechanism, surface chemistry and catalyst formulation. Biofuels Eng 2:11–31. https://doi.org/10.1515/bfuel-2017-0002
Borg Ø, Eri S, Blekkan EA, Storsæter S, Wigum H, Rytter E, Holmen A (2007) Fischer-Tropsch synthesis over γ-alumina-supported cobalt catalysts: effect of support variables. J Catal 248:89–100. https://doi.org/10.1016/j.jcat.2007.03.008
Borg Ø, Dietzel PDC, Spjelkavik AI, Tveten EZ, Walmsley JC, Diplas S, Eri S, Holmen A, Rytter E (2008) Fischer-Tropsch synthesis: cobalt particle size and support effects on intrinsic activity and product distribution. J Catal 259:161–164. https://doi.org/10.1016/j.jcat.2008.08.017
Rane S, Borg Ø, Yang J, Rytter E, Holmen A (2010) Effect of alumina phases on hydrocarbon selectivity in Fischer-Tropsch synthesis. Appl Catal A 388:160–167. https://doi.org/10.1016/j.apcata.2010.08.038
Rane S, Borg Ø, Rytter E, Holmen A (2012) Relation between hydrocarbon selectivity and cobalt particle size for alumina supported cobalt Fischer-Tropsch catalysts. Appl Catal A 437–438:10–17. https://doi.org/10.1016/j.apcata.2012.06.005
Xiong H, Zhang Y, Wang S, Li J (2005) Fischer-Tropsch synthesis: the effect of Al2O3 porosity on the performance of Co/Al2O3 catalyst. Catal Commun 6:512–516. https://doi.org/10.1016/j.catcom.2005.04.018
Borg Ø, Hammer N, Eri S, Lindvåg OA, Myrstad R, Blekkan EA, Rønning M, Rytter E, Holmen A (2009) Fischer-Tropsch synthesis over un-promoted and Re-promoted γ-Al2O3 supported cobalt catalysts with different pore sizes. Catal Today 142:70–77. https://doi.org/10.1016/j.cattod.2009.01.012
Rytter E, Borg Ø, Tsakoumis NE, Holmen A (2018) Water as key to activity and selectivity in Co Fischer-Tropsch synthesis: y-alumina based structure-performance relationships. J Catal 365:334–343. https://doi.org/10.1016/j.jcat.2018.07.003
Rytter E, Borg Ø, Enger BC, Holmen A (2019) Α-alumina as catalyst support in Co Fischer-Tropsch synthesis and the effect of added water; encompassing transient effects. J Catal 373:13–24. https://doi.org/10.1016/j.jcat.2019.03.013
Amirov N, Vakhshouri AR (2020) Numerical modeling and optimization of product selectivity and catalyst activity in Fischer-Tropsch synthesis via response surface methodology: cobalt carbide particle size and H2/CO ratio effects. Int J Hydrogen Energy 45(56):31913–31925. https://doi.org/10.1016/j.ijhydene.2020.08.219
Ghattavi S, Nezamzadeh-ejhieh A (2020) GC-MASS detection of methyl orange degradation intermediates by AgBr/g-C3N4: experimental design, bandgap study, and characterization of the catalyst. Int J Hydrogen Energy. https://doi.org/10.1016/j.ijhydene.2020.06.207
Derikvandi H, Nezamzadeh-ejhieh A (2016) Synergistic effect of p-n heterojunction, supporting and zeolite nanoparticles in enhanced photocatalytic activity of NiO and SnO2. J Colloid Interface Sci 490:314–327. https://doi.org/10.1016/j.jcis.2016.11.069
Atashi H, Veiskarami S (2019) Determination of models and optimization for the products of Fischer-Teropsch synthesis on Fe-Mn/K catalyst. Pet Sci Technol 37:1960–1967. https://doi.org/10.1080/10916466.2018.1471503
Atashi H, Veiskarami S (2018) Green fuel from coal via Fischer-Tropsch process: scenario of optimal condition of process and modelling. Int J Coal Sci Technol 5:230–243. https://doi.org/10.1007/s40789-018-0204-7
Atashi H, Rezaeian F (2017) Modelling and optimization of Fischer-Tropsch products through iron catalyst in fixed-bed reactor. Int J Hydrogen Energy 42:15497–15506. https://doi.org/10.1016/j.ijhydene.2017.04.224
Atashi H, Dinarvandi K (2019) Determination of selectivity equations heavy and light product of petroleum on iron based catalysts in Fischer-Tropsch synthesis. Pet Sci Technol 37:2035–2042. https://doi.org/10.1080/10916466.2018.1460610
Riyahin M, Atashi H, Mohebbi-Kalhori D (2016) Effect of process conditions on Fischer-Tropsch synthesis product selectivity over an industrial iron-based catalyst in slurry reactor. Pet Sci Technol 34:1211–1218. https://doi.org/10.1080/10916466.2016.1193521
Riyahin M, Mohebbi-Kalhori D, Zohdi-Fasaei H, Mirzaei AA, Atashi H (2020) Proposing innovative modeling for Fischer-Tropsch synthesis product selectivity over cobalt catalyst and skewness analyzing. Pet Sci Technol 38:1–9. https://doi.org/10.1080/10916466.2019.1705859
Myers RH, Montgomery DC, Anderson-Cook CM (2009) Response surface methodology: process and products optimization using designed experiments, 3rd edn. Wiley, New York
Support.minitab.com. (2019) Minitab 18 support: Minitab. https://support.minitab.com/en-us/minitab/18/.
Eshraghi F, Nezamzadeh-ejhieh A (2018) EDTA-functionalized clinoptilolite nanoparticles as an effective adsorbent for Pb(II) removal. Environ Sci Pollut Res 25:14043–14056. https://doi.org/10.1007/s11356-018-1461-0
State-Ease, Inc. (2021) Stat-ease handbook for experimenters. State-Ease Inc, Minneapolis
Omrani N, Nezamzadeh-Ejhieh A (2020) Photodegradation of sulfasalazine over Cu2O-BiVO4-WO3 nano-composite: characterization and experimental design. Int J Hydrog 45:19144–19162. https://doi.org/10.1016/j.ijhydene.2020.05.019
Tamiji T, Nezamzadeh-Ejhieh A (2018) A comprehensive study on the kinetic aspects and experimental design for the voltammetric response of a Sn(IV)-clinoptilolite carbon paste electrode towards Hg(II). J Electroanal Chem 829:95–105. https://doi.org/10.1016/j.jelechem.2018.10.011
Xu X, Oosterbeek H, de Bitter JH, van Jong KP, Dillen AJ, Kuipers HPCE, Bezemer GL, Holewijn JE, Kapteijn F (2006) Cobalt particle size effects in the Fischer−Tropsch reaction studied with carbon nanofiber supported catalysts. J Am Chem Soc 128:3956–3964. https://doi.org/10.1021/ja058282w
Rytter E, Borg Ø, Tsakoumis NE, Holmen A (2018) Water as key to activity and selectivity in Co Fischer-Tropsch synthesis : γ-alumina based structure-performance relationships. J Catal 365:334–343. https://doi.org/10.1016/j.jcat.2018.07.003
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amirov, N., Vakhshouri, A.R. Statistical Product Selectivity Modeling and Optimization for γ-Al2O3-Supported Cobalt Catalysts-Based Fischer–Tropsch Synthesis. Catal Lett 151, 3273–3286 (2021). https://doi.org/10.1007/s10562-021-03557-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03557-0