Abstract
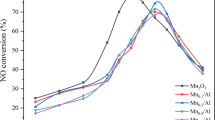
Different crystal phases of MnO2 were synthesized and tested for NH3–SCR of NO and NH3 oxidation performances during 50–120 °C. Among those catalysts, α-MnO2 showed the most superior SCR performance for NOx conversion and N2 selectivity, and NH3 species on its surface were active to react with the oxygen, while NH2 species were also easily oxidized by the oxygen. For β-MnO2, NH3 (ads, B) species and part of NH3 (ads, L) species on its surface were active to react with oxygen, while NH3 (ads, L) species adsorbed at Lewis sites showed low reactive with O2, thereby producing less N2O and low NO conversion. γ-MnO2 showed the similar NOx conversion rates and N2O amount generated from the NH3 oxidation comparing to α-MnO2, while yielding much more N2O generation ratios from SCR reactions conditions. Besides, NH3 (ads, L), NH3 (ads, B), NO32− and NH2 species adsorbed on γ-MnO2 surface had high reactivity and could all be consumed by oxygen rapidly. And the N2O formation of δ-MnO2 mainly generated from SCR reactions conditions in the temperature range of 50–120 °C, while the adsorbed NH3 (ads, L) species were hard to react with O2.
Graphic Abstract






Similar content being viewed by others
References
Gao C, Xiao B, Shi JW et al (2019) Comprehensive understanding the promoting effect of Dy-doping on MnFeOx nanowires for the low-temperature NH3-SCR of NOx: an experimental and theoretical study. J Catal 380:55–67
Chen H, Wang Y, Lyu YK et al (2019) In-situ DRIFTS for reaction mechanism and SO2 poisoning mechanism of NO oxidation over γ-MnO2 with good low-temperature activity. Catal Lett 149:753–765
Damma D, Ettireddy PR, Reddy BM et al (2019) A review of low temperature NH3-SCR for removal of NOx. Catalysts 9(4):349
Krocher O (2018) Selective Catalytic Reduction of NOx. Catalysts 8(10):459
Ren S, Aldahri T, Liu WZ et al (2021) CO2 mineral sequestration by using blast furnace slag: from batch to continuous experiments. Energy 214:118975
Schneider H, Maciejewski M, Kohler K et al (1995) Chromia supported on titania: properties of different chromium oxide phases in the catalytic reduction of NO by NH3 studied by in situ diffuse reflectance FTIR spectroscopy. J Catal 157(2):312–320
Li R, Wu B, Chen YQ et al (2019) Infuence of polyethylene glycol on the catalytic activity of MnFeOx for NO oxidation at low-temperature. Catal Lett 149:1864–1873
Sreekanth PM, Pena DA, Smirniotis PG (2006) Titania supported bimetallic transition metal oxides for low-temperature SCR of NO with NH3. Ind Eng Chem Res 45(19):6444–6449
Yang J, Zhou J, Tong W et al (2019) Low-temperature flue gas denitration with transition metal oxides supported on biomass char. J Energy Inst 92(4):1158–1166
Xin Y, Li H, Zhang NN et al (2018) Molecular-level insight into selective catalytic reduction of NOx with NH3 to N2 over a highly efficient bifunctional V: alpha-MnOx catalyst at low temperature. ACS Catal 8(6):4937–4949
Kang M, Park ED, Kim JM et al (2007) Manganese oxide catalysts for NOx reduction with NH3 at low temperatures. Appl Catal A 327(2):261–269
Li JH, Chang HZ, Ma L et al (2011) Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts: a review. Catal Today 175(1):147–156
Ren S, Yang J, Zhang TS et al (2018) Role of cerium in improving NO reduction with NH3 over Mn-Ce/ASC catalyst in low-temperature flue gas. Chem Eng Res Des 133:1–10
Yang J, Ren S, Zhang TS et al (2020) Iron doped effects on active sites formation over activated carbon supported Mn-Ce oxide catalysts for low-temperature SCR of NO. Chem Eng J 379:122398
Yao L, Liu QC, Mossin S et al (2020) Promotional effects of nitrogen doping on catalytic performance over manganese-containing semi-coke catalysts for the NH3-SCR at low temperatures. J Hazard Mater 387:121704
Jiang LJ, Liu QC, Ran GJ et al (2019) V2O5-modified Mn-Ce/AC catalyst with high SO2 tolerance for low-temperature NH3-SCR of NO. Chem Eng J 370:810–821
Ilchenko NI, Golodets GI (1975) Catalytic-oxidation of ammonia. 1. Reaction-kinetics and mechanism. J Catal 39(1):57–72
Ilchenko NI, Golodets GI (1975) Catalytic-oxidation of ammonia 2: relationship between catalytic properties of substances and surface oxygen bond-energy - general regularities in catalytic-oxidation of ammonia and organic-substances. J Catal 39(1):73–86
Zhu MH, Lai JK, Wachs IE (2018) Formation of N2O greenhouse gas during SCR of NO with NH3 by supported vanadium oxide catalysts. Appl Catal B 224:836–840
Yang J, Su ZH, Ren S et al (2019) Low-temperature SCR of NO with NH3 over biomass char supported highly dispersed Mn-Ce mixed oxides. J Energy Inst 92(4):883–891
Huang JZ, Zhang HC, Zhong SF (2018) Effect of MnO2 phase structure on the oxidative reactivity toward bisphenol a degradation. Abstr Pap Am Chem 52:11309
Chen BB, Wu B, Yu LM et al (2020) Investigation into the catalytic roles of various oxygen species over different crystal phases of MnO2 for C6H6 and HCHO oxidation. ACS Catal 110(11):6176–6187
Yang WH, Su ZA, Xu ZH et al (2020) Comparative study of α-, β-, γ- and σ-MnO2 on toluene oxidation: oxygen vacancies and reaction intermediates. Appl Catal B 260:118150
Zhang JH, Li YB, Wang L et al (2015) Catalytic oxidation of formaldehyde over manganese oxides with different crystal structures. Catal Sci Technol 5(4):2305–2313
Xin Y, Zhang NN, Li Q et al (2018) Selective catalytic reduction of NOx with NH3 over short-range ordered W-O-Fe structures with high thermal stability. Appl Catal B 229:81–87
Wang H, Chen H, Wang Y et al (2019) Performance and mechanism comparison of manganese oxides at different valence states for catalytic oxidation of NO. Chem Eng J 361:1161–1172
Zhu MH, Lai JK, Tumuluri U et al (2017) Nature of active sites and surface intermediates during SCR of NO with NH3 by supported V2O5-WO3/TiO2 catalysts. J Am Chem Soc 139(44):15624–15627
Gao FY, Tang XL, Yi HH et al (2017) Promotional mechanisms of activity and SO2 tolerance of Co- or Ni-doped MnOx-CeO2 catalysts for SCR of NOx with NH3 at low temperature. Chem Eng J 317:20–31
Liu FD, He H, Zhang CB et al (2011) Mechanism of the selective catalytic reduction of NOx with NH3 over environmental-friendly iron titanate catalyst. Catal Today 175(1):18–25
Kong M, Liu QC, Zhou J, Jiang LJ, Tian YM, Yang J et al (2018) Effect of different potassium species on the deactivation of V2O5-WO3/TiO2 SCR catalyst: comparison of K2SO4, KCl and K2O. Chem Eng J 348:637–643
Yang SJ, Xiong SC, Liao Y, Xiao X, Qi FH, Peng Y et al (2014) Mechanism of N2O formation during the low-temperature selective catalytic reduction of NO with NH3 over Mn-Fe spinel. Environ Sci Technol 48(17):10354–10362
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 51874058), Chongqing Talents Plan for Young Talents (CQYC201905017), Graduate Research and Innovation Foundation of Chongqing, China (No. CYB20003) and Fund of Chongqing Science and Technology (cstc2019jscx-msxmX0215 and cstc2019jscx-fxydX0009).
Author information
Authors and Affiliations
Contributions
JY: Conceptualization, Methodology, Data Curation, Writing-Original draft preparation, Formal analysis, Investigation; SR: Conceptualization, Methodology, Investigation, Writing-Reviewing & Editing, Funding acquisition, Supervision; BS: Writing-Reviewing; YZ: Formal analysis, Writing-Original draft preparation, Investigation; GH: Formal analysis; LJ: Visualization; JC: Investigation; WL: Validation, Data curation; LY: Validation, Data curation; MK: Formal analysis, Writing-Reviewing & Editing; JY: Formal analysis, Writing–Reviewing & Editing; QL: Supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yang, J., Ren, S., Su, B. et al. Insight into N2O Formation Over Different Crystal Phases of MnO2 During Low-Temperature NH3–SCR of NO. Catal Lett 151, 2964–2971 (2021). https://doi.org/10.1007/s10562-021-03541-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-021-03541-8